The concepts of “mole” and “atom” serve as foundational pillars in the realm of chemistry, offering a framework that allows for the quantification and manipulation of matter at a microscopic scale. Although these terms are interrelated, they represent distinct entities in the scientific lexicon that merit a thorough exploration to illuminate their differences and interdependencies.
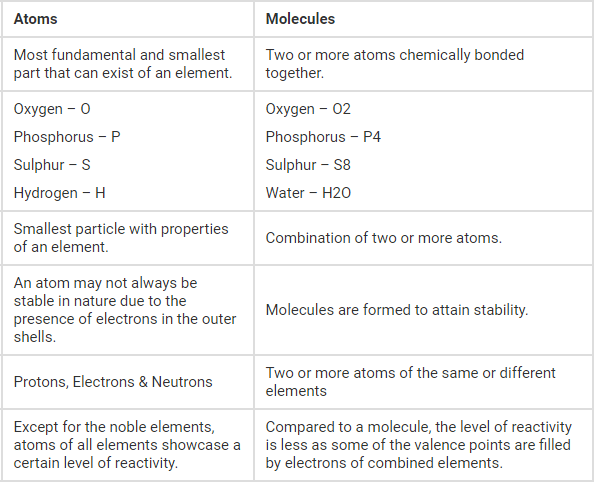
To begin with, it is essential to define what an atom is. An atom is the most fundamental building block of matter, composed of a nucleus surrounded by electrons. The nucleus itself contains protons and neutrons, which together account for the majority of an atom’s mass. Atoms can exist independently or combine with others to form molecules, thereby engaging in myriad chemical reactions that contribute to the complexity of the material world. Each chemical element is represented by a unique type of atom, characterized by a specific number of protons, known as the atomic number.
On the other hand, the “mole” is a unit of measurement in the International System of Units (SI) that quantifies the amount of substance. It is not a substance itself but a way to describe a quantity of atoms, molecules, or ions. One mole of any substance contains approximately (6.022 times 10^{23}) entities, a figure known as Avogadro’s number. This numerical constant serves as a bridge between the atomic scale and the macroscopic quantities we experience in the laboratory and everyday life.
The dichotomy between these two concepts becomes even more pronounced when considering their applicability in various chemical contexts. For example, the notion of a mole allows chemists to scale their operations—from tiny reactions involving individual atoms to reactions involving macroscopic samples measured in grams. This scaling is crucial for practical laboratory work, where precise measurements often dictate the outcome of experiments. In essence, while an atom represents the constituent parts of matter, a mole encapsulates the idea of a manageable quantity of these parts.
It is vital to appreciate the significance of the mole in stoichiometric calculations. Stoichiometry involves the calculation of reactants and products in chemical reactions—a process that relies heavily on the mole concept to determine how much of each substance is required or produced. When balanced chemical equations are employed, the coefficients indicate the ratio of moles of reactants to moles of products, thereby enabling chemists to precisely predict the outcomes of reactions.
Moreover, the mole is particularly useful in the context of molarity, a commonly used unit of concentration that expresses the amount of solute in a given volume of solution. This is integral to the preparation of solutions and conducting reactions in which concentration plays a crucial role. For instance, when preparing a dilute acid solution from a concentrated stock, knowing the molar concentration allows one to calculate the required volume accurately, thus ensuring safety and efficacy in chemical procedures.
Furthermore, it is intriguing to consider how the differences between moles and atoms extend into the subtleties of chemical reactivity and bonding. Atoms tend to bond with one another, resulting in the formation of molecules, which can exist in numerous structural forms. A molecule, whether it’s a simple diatomic gas like ( text{H}_2 ) or a complex macromolecule like DNA, is also represented in terms of moles. Understanding this duality only emphasizes the relevance of both atoms and moles in the broader tapestry of chemical understanding.
Another captivating dimension arises when one examines the concept of molar mass. Molar mass is the mass of one mole of a substance expressed in grams per mole (g/mol). This concept can be particularly helpful in converting between mass and mole quantities, evoking a fascinating interplay between atomic scale properties and everyday measurements. For instance, a chemist might determine the molar mass of glucose (C₆H₁₂O₆) to be roughly 180 g/mol, which implies that one mole of glucose weighs 180 grams. This tangible connection showcases how invisible atoms translate into measurable quantities that we can manipulate in the laboratory.
As we delve deeper into the microscopic world governed by these concepts, it becomes apparent that both atoms and moles are indispensable for deciphering the intricate dance of chemical phenomena. Whether one is contemplating the simplicity of a single atom or the complexity of a molar quantity, the interplay between these two ideas fosters a greater understanding of the natural world.
In conclusion, while atoms serve as the elemental constituents of matter, moles offer a practical method to quantify and work with these atoms on a scale that is both manageable and meaningful. The differences between these two terms underscore a deeper understanding of chemistry and the methodologies that scientists utilize to explore and manipulate the world around us. Grasping these concepts prepares the field for innovation and inspires curiosity about the limitless possibilities that await in the exploration of matter.