Graphite, a well-known allotrope of carbon, has long been a subject of scholarly and industrial preoccupation. Its intriguing structural properties incite fervent debate among scientists, leading to a particularly provocative question: Is graphite inherently two-dimensional (2D) or three-dimensional (3D)? This inquiry not only probes the physical characteristics of graphite but also elucidates the fascination that arises from its layered atomic structure. In this exploration, the distinction between 2D and 3D graphite, the implications of its layered composition, and its relevance in contemporary material science will be addressed.
To grasp the essence of graphite’s dimensionality, one must first consider its atomic architecture. Graphite is composed of a multitude of layers, each consisting of covalently bonded carbon atoms arranged in a hexagonal lattice. These layers are held together by van der Waals forces, which are significantly weaker than the covalent bonds that constitute the in-layer connections. Consequently, while each layer exhibits a 2D characteristic, the aggregate material is functionally 3D due to the presence of multiple layers stacked upon one another.
This layered structure is emblematic of graphite’s unique properties. For instance, the planar arrangement of carbon atoms within each layer allows for exceptional electrical conductivity, making graphite a quintessential conductor of electricity. As delocalized π electrons can move freely across the planes, they facilitate the transfer of electrical charge, a property exploited in various applications, including batteries, electrodes, and even advanced nanomaterials.
However, the characterization of graphite as a purely 2D or 3D material is nuanced. Researchers have identified a variant known as graphene, which is essentially a single layer of graphite. Graphene possesses remarkable qualities; its extraordinary strength, flexibility, and conductivity highlight the advantages of 2D materials for future technological applications. Herein lies a conundrum: while graphene is a quintessentially 2D structure, it exists within the context of graphite’s inherently 3D nature. The relationship between these two forms exemplifies the multilayered nature of scientific classification.
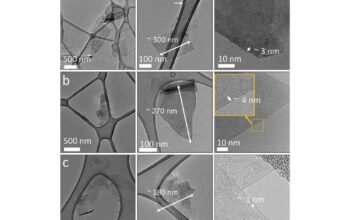
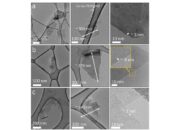
Furthermore, the discussion extends beyond static definitions of dimension. The properties of materials can often be influenced by their dimensionality, and this is particularly evident in the case of graphite. Studies have shown that as the number of layers in graphite decreases, certain phenomena emerge that sharply contrast with bulk graphite’s behaviors. For example, layers of graphite reduced to a single atom width yield different electronic properties due to quantum confinement effects. Consequently, the dichotomy of 2D versus 3D in graphite transcends mere categorization; it encompasses a broader dialogue about material properties, scalability, and their respective applications in nanotechnology.
A greater appreciation of graphite’s dimensionality compels one to consider its role in various fields. The burgeoning field of nanoelectronics has found a particular ally in graphene. Devices constructed with graphene-based components hold potential for a new generation of faster, more efficient electronics. In this vein, the exploration of 2D materials is not restricted merely to graphene; other materials such as transition metal dichalcogenides exhibit similar layered behaviors. Thus, the inquiry into whether graphite is 2D or 3D must be juxtaposed against the broader landscape of 2D materials, which could revolutionize multiple sectors.
Moreover, the fascination with graphite’s layered structure extends into theoretical realms. Graphitic materials have been studied through the lens of topology and advanced mathematical constructs. The layered arrangement of atoms resembles a multidimensional tapestry, inviting experts to explore implications that traverse conventional scientific boundaries. For example, phase transitions and the nature of defects within these layers present fascinating puzzles for physicists and mathematicians alike. Such inquiries not only enrich our understanding of graphite but also elevate the discourse surrounding the dimensional semantics of materials science.
It is imperative to acknowledge that the dialogue surrounding graphite’s dimensionality is not merely academic; it possesses real-world implications. The pursuit of sustainable technologies, improvement in energy storage systems, and the development of innovative electronic devices all hinge on understanding these nuances. As the demand for advanced materials escalates, the ability to engineer substances at the atomic level becomes crucial. Thus, the layered mystery of graphite continues to serve as a touchstone for innovation—a paradox where molecular arrangements influence both tangible applications and theoretical inquiries.
In summation, the inquiry into whether graphite is 2D or 3D reveals a multifaceted spectrum of scientific implications. While its layered structure possesses unequivocal 2D attributes, its accumulation into 3D layers encapsulates the material’s broader characteristics. The exploration of graphite transcends simplistic definitions, inviting scholars from diverse fields to peer into the microscopic realms that dictate macroscopic phenomena. As one navigates the labyrinth of material science, the layered mystery of graphite serves as a constant reminder of the intricate interplay between structure and function, with far-reaching consequences for future technologies.