Graphene, a remarkable allotrope of carbon, has eluded the paradigms of conventional material science since its revelation. It is essential to elucidate the intrinsic characteristics of graphene and its relationship with graphite, particularly addressing the assertion that graphene constitutes a single layer of graphite. The subsequent discourse aims to elaborate on the nature of graphene, contrasting it with graphite, while examining its properties, applications, and implications in diverse scientific and technological realms.
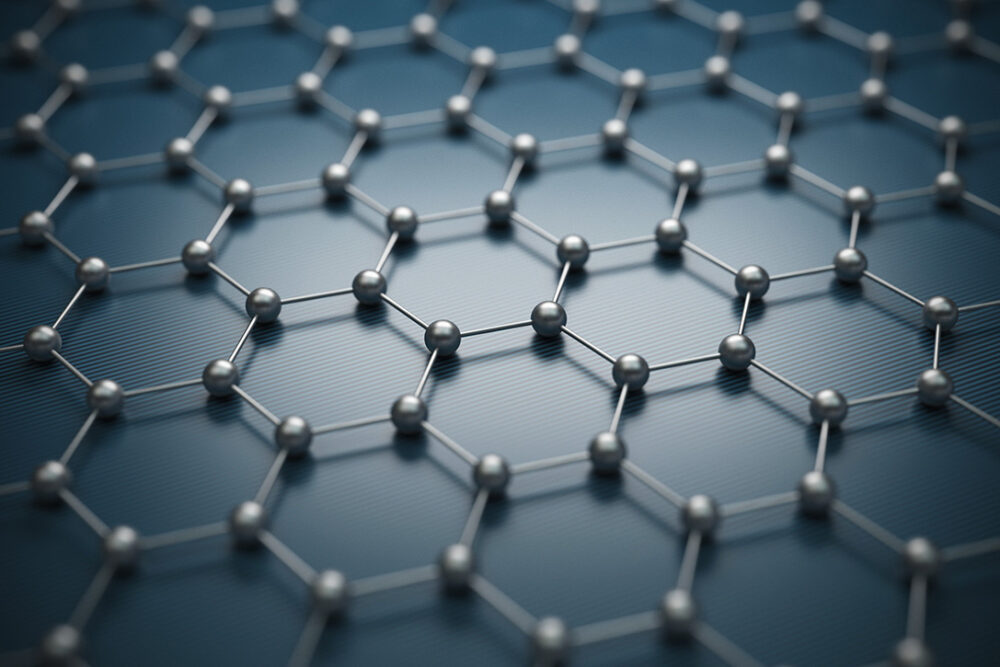
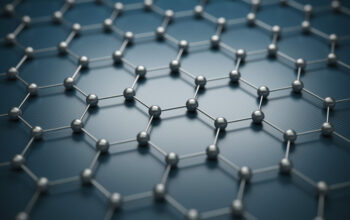
Graphene is comprised of a two-dimensional arrangement of carbon atoms, possessing an extraordinary array of properties that differentiate it from its parent material, graphite. To comprehend the distinction, one must first recognize the structural differences. Graphite is a macroscopic form of carbon that consists of numerous layers of graphene stacked atop one another through weak van der Waals forces. These layers confer specific bulk properties to graphite, such as its lubricity and electrical conductivity. In contrast, a single layer of graphene is defined as a monolayer of carbon atoms organized in a hexagonal lattice, yielding remarkable characteristics, including high tensile strength, exceptional electrical conductivity, and significant thermal conductivity.
This unique hexagonal arrangement allows each carbon atom to hybridize through sp2 bonding, forming three sigma bonds with adjacent carbon atoms while leaving a p-orbital electron delocalized. This delocalization creates a pi-bonding network that is responsible for the remarkable electronic properties of graphene. One is compelled to inquire how these properties can manifest intangible benefits to real-world applications. The answer lies in the material’s potential to revolutionize various fields ranging from electronics to biomedicine.
In electronics, graphene has generated considerable excitement due to its unparalleled electrical conductivity. The electron mobility in graphene is significantly higher than that of silicon, making it a strong candidate for the next generation of transistors. Graphene-based field-effect transistors (FETs) could permit the development of faster-processing units, reducing energy consumption in electronic devices while enhancing performance metrics. However, the integration of graphene into current semiconductor technologies remains impeded by challenges in bandgap engineering, which is critical for switching applications. Ongoing research seeks to selectively manipulate the electronic properties of graphene to facilitate its incorporation into existing electronic frameworks.
Furthermore, the potential of graphene extends into the realm of energy storage. Its high surface area and conductivity yield promising applications for supercapacitors and batteries. Graphene can enhance the charge and discharge rates in supercapacitors, providing a solution to the limitations imposed by conventional capacitors. Notably, the use of graphene as an anode material in lithium-ion batteries has shown advancements in increasing energy density and lifecycle. The implications for electric vehicles and renewable energy systems are profound, as efficient energy storage mechanisms are paramount for reducing carbon footprints and enhancing sustainability.
The unique molecular structure of graphene does not confine its potential solely to electronic applications, but also envelops the field of materials science. Due to its mechanical properties, graphene exhibits exceptional tensile strength; it is theoretically hundreds of times stronger than steel while remaining lightweight and flexible. This characteristic introduces opportunities for creating composite materials that are both strong and lightweight, fostering advancements in aerospace engineering, construction, and nanotechnology. Such composites could lead to the development of materials that are resilient and resistant to various forms of stress and degradation.
Moreover, the optical properties of graphene are captivating, exhibiting transparency coupled with effective confinement of electromagnetic radiation. Researchers are exploring its application in photonics, where graphene can be utilized in devices such as photodetectors, modulators, and transparent conductive films. As the demand for efficient and versatile optoelectronic devices grows, graphene’s role could become even more pivotal, particularly in developing high-speed communication technologies.
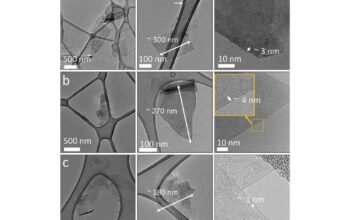
Despite the myriad of advantages graphene presents, the route toward mass production and commercialization is fraught with complexities. Techniques such as mechanical exfoliation, chemical vapor deposition (CVD), and liquid-phase exfoliation are employed to synthesize graphene. However, achieving uniform properties across large areas of graphene remains a challenge. Moreover, the scalability of these production techniques, alongside economic viability, must be resolved to facilitate widespread adoption in various industries.
Lastly, the implications of graphene extend into the biomedical sphere. Its biocompatibility and antimicrobial properties render it suitable for various applications, including drug delivery, biosensors, and tissue engineering scaffolds. Researchers are investigating graphene’s potential to devise innovative therapeutic approaches and diagnostics, potentially altering paradigms in healthcare.
In conclusion, while graphene is fundamentally a single layer of graphite, its ramifications are vast and multifaceted. Its exceptional properties herald the dawn of a new era of materials with transformative potential across a variety of scientific domains. As researchers continue to unravel the complexities surrounding graphene’s production, integration, and application, the quest to harness this material may well redefine technological landscapes, prompting paradigm shifts that align with the exigencies of a rapidly evolving world.