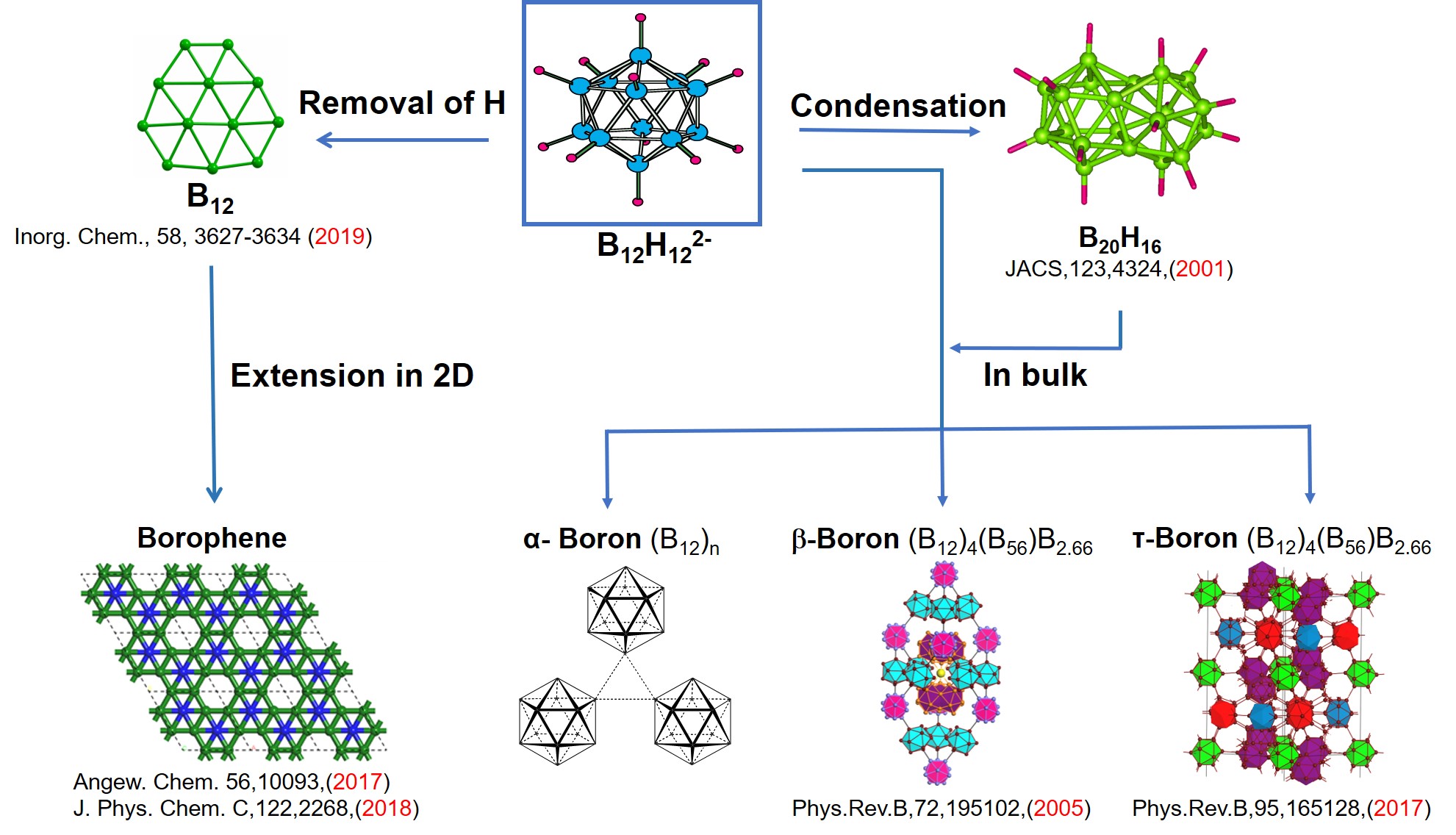
Boron, an intriguing metalloid with the atomic number 5, presents a plethora of remarkable properties that have captured the attention of researchers and academics alike. However, one characteristic that frequently eludes thorough comprehension is its status as a poor conductor of electricity. This article seeks to explore the reasons behind boron’s inefficacy as a conductor, aiming to untangle a web of atomic structure, bonding behavior, and electronic configuration. So, what makes boron a less-than-ideal conductor? Let us delve into this captivating conundrum.
First and foremost, it is crucial to understand the electronic configuration of boron, which is 1s² 2s² 2p¹. With only three valence electrons, the distribution reveals a significant limitation in its ability to facilitate electrical conduction. Conductivity, the measure of a material’s ability to transmit electric current, largely depends on the availability and mobility of charge carriers—primarily free electrons. In a metal, a surplus of free electrons readily moves through the lattice structure, facilitating conduction. However, boron’s insufficient valence electrons result in a scarcity of free charge carriers.
The intrinsic properties of the covalent bonds formed between boron atoms offer further insight into its conductive limitations. When boron atoms bond, they tend to form strong covalent interactions due to their non-metallic nature. This bonding is characterized by a tetrahedral arrangement against a backdrop of hybridized orbitals (sp³) which enhances stability at the expense of conductivity. The formation of such covalent bonds leads to the creation of a rigid lattice that effectively inhibits the motion of electrons, thus constraining the conduction path.
Moreover, unlike metals, which showcase partially filled d-orbitals contributing to the conduction process, boron lacks any utilization of d-orbitals in its basic conductive behavior. This absence restricts the availability of conduction bands, leaving the conduction mechanism significantly impaired. While boron can exhibit some level of conductivity under specific conditions, particularly when doped with other elements in semiconductor applications, its intrinsic properties still dictate a general framework of limited conductivity.
Now, ponder this: What would happen if one attempted to advance the conductivity of boron significantly? Would introducing impurities or employing unique alloying techniques alter this characteristic? Indeed, in the realm of materials science, such innovations warrant exploration. Boron’s conductivity can be enhanced through the incorporation of dopants, yielding interesting results. For instance, when boron is doped with elements like phosphorus or arsenic, it shifts into the realm of semiconductors, where electrical conductivity can be fine-tuned with precision.
Additionally, the crystalline structure of boron also plays a pivotal role in its ability to conduct electricity. Boron is known to crystallize in several allotropes, including amorphous and crystalline forms. The crystalline variations exhibit distinct atomic arrangements, influencing their electronic properties and conductivity. The crystalline form, specifically, can potentially offer avenues for enhanced conductivity when engineered appropriately. Yet, even within these structures, boron’s unique electronic properties continue to grapple with the prevalent challenges posed by its bonding characteristics.
Furthermore, it is essential to recognize the temperature dependence of boron’s conductivity. As temperature rises, the intrinsic carrier concentration tends to increase due to thermal excitation. This effect is pivotal for semiconductors, where temperature fluctuations can lead to enhanced conductivity. However, boron, even in its doped forms, will still exhibit inferior conductivity compared to its metallic counterparts, highlighting the inherent limitations imposed by its atomic constitution.
The analysis of boron as a poor conductor naturally leads to comparisons with other non-metals and metalloids, shedding light on diverse conductive properties found within this group. For instance, silicon, a renowned semiconductor, displays enhanced conductivity due to its distinctive crystalline structure and the presence of additional valence electrons. In contrast to boron, silicon possesses four valence electrons, allowing for more robust bonding configurations and increased charge carrier mobility. Thus, boron finds itself often overshadowed in the realm of conductivity by its cohort metalloids and the metals themselves.
In summary, the investigation of boron as a bad conductor reveals a fascinating interplay between atomic structure, bonding characteristics, and electronic properties. With its limited valence electron capacity and predominant covalent bonding behavior, boron inherently struggles to offer the same electrical conductivity as many metals. However, the potential for enhancement through doping and innovative materials science poses an intriguing question for further exploration. Can we architect a boron-based material that rivals the conductivity of traditional conductors? The intersection of theoretical exploration and practical application in this domain holds remarkable promise, meriting deeper inquiry into the fundamental aspects of boron’s conductive capabilities.
In closing, the reasons underlying boron’s inability to conduct electricity effectively are multifaceted, spanning atomic structure, bonding characteristics, and the profound influence of temperature and doping. While boron inherently possesses conductive limitations, its unique properties beg further inquiry. Whether through innovative materials science approaches or a deeper understanding of its electronic behavior, boron remains a compelling subject within the realm of conductivity, fostering ongoing curiosity and investigation.