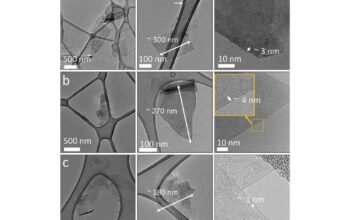
Graphene—a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice—has captured the attention of researchers, material scientists, and industries alike, thanks to its extraordinary properties. Among these, the intriguing assertion that graphene can exhibit hardness surpassing that of diamond invites a deeper exploration into the fundamental nature of hardness and the physics underlying these materials.
Diamond, renowned as the hardest natural material known to mankind, has long been associated with durability and resilience. Its tetrahedral lattice structure, where each carbon atom covalently bonds with four others, imbues it with remarkable strength and rigidity. Yet, this perception invites a critical examination: hardness is not merely an intrinsic property but is profoundly influenced by the material’s structural characteristics, bonding mechanisms, and the geometrical arrangement of its constituent atoms.
To elucidate how graphene presents a fascinating juxtaposition to diamond, one must first comprehend what defines hardness in materials. Generally characterized as a material’s resistance to deformation, particularly permanent deformation, hardness can be quantified through various methodologies, including the Mohs scale and Vickers hardness tests. At face value, diamond dominates these scales, achieving a perfect 10 on Mohs. However, hardness does not exist in isolation; it is also a function of factors such as elasticity, tensile strength, and the mechanism of stress distribution within a material.
Graphene, while drastically thinner than a diamond, exhibits unique mechanical properties that stem from its two-dimensional nature. This structure enables graphene to manifest a tensile strength exceeding that of steel, estimated to be around 200 gigapascals. The nature of graphene’s carbon-carbon bonds—a hybridization of sp2—yields an exceptional combination of strength and flexibility. When subjected to stress, graphene can effectively redistribute applied forces across its lattice, thereby mitigating local points of weakness. This phenomenon of stress distribution is integral to understanding why, at the nanoscale, graphene can outperform diamond under certain conditions.
Moreover, the concept of hardness itself is contingent upon the interaction of a material with an external force. The hardness of diamond is attributable to its rigid lattice structure; however, this rigidity also renders it susceptible to fracturing under certain circumstances. On the contrary, the flexibility inherent in graphene’s two-dimensional form allows it to withstand substantial deformation without fracturing. Experimentally, graphene has been shown to endure strains of up to 25% before yielding, a feat that diamonds cannot achieve without experiencing catastrophic failure.
This ability to maintain structural integrity under duress leads to the introduction of another critical parameter: toughness. While hardness typically addresses resistance to scratching or indentation, toughness encompasses a material’s ability to absorb energy and plastically deform without fracturing. In this context, graphene’s capacity for elongation and resilience in the face of mechanical stress marks a distinct advantage over diamond. The interplay of intrinsic strength and the capability to undergo reversible deformation positions graphene not merely as a harder material in certain aspects, but as a fundamentally superior candidate for applications that necessitate both hardness and resilience.
Furthermore, the implications of graphene’s exceptional properties extend beyond academic inquiry, as they promise revolutionary advancements across various fields. In nanotechnology, for example, the production of graphene-based composites could yield materials that surpass the limitations of conventional substances. Graphene-infused polymers could be engineered to produce lighter, stronger, and more resilient materials, propelling innovations in aerospace, automotive, and even consumer electronics. The potential of graphene should incite curiosity; it is not merely a material but a gateway to redefining our understanding of solidity and durability in engineering.
The confluence of hardness and flexibility heralds potential developments in biomedical applications as well. The biocompatibility of graphene, coupled with its mechanical robustness, positions it as an innovative candidate for prosthetics, implants, and even tissue scaffolds. Here, the key is not only hardness but the ability to integrate seamlessly with biological systems while maintaining functionality over extended periods.
Future studies aim to explore the scalability of graphene’s production and optimization of its mechanical properties through various chemical modifications. Techniques such as doping or the creation of graphene oxide derivatives could pave the way for engineering materials tailored for specific applications, allowing for a fine-tuned manipulation of hardness without compromising other essential characteristics.
The narrative surrounding hardness and the comparative analysis of graphene and diamond invites an essential reconsideration of defined material paradigms. In transcending the traditional understanding of what constitutes ‘hardness,’ one encounters exciting inquiries into the evolving landscape of materials science. It is through such explorations that the boundaries of technology and nature may intertwine, revealing not only new materials but also fresh perspectives on the physical world.
In conclusion, the discourse on whether graphene can indeed be considered ‘harder’ than diamond is complex, nuanced, and deeply rooted in materials physics. Through structural analysis, stress distribution capabilities, and a broader interpretation of toughness, researchers reaffirm that hardness is multifaceted and context-dependent. Consequently, the ongoing research into graphene asserts its potential as a transformative material, stirring anticipation and curiosity about its future applications and implications across disciplines.