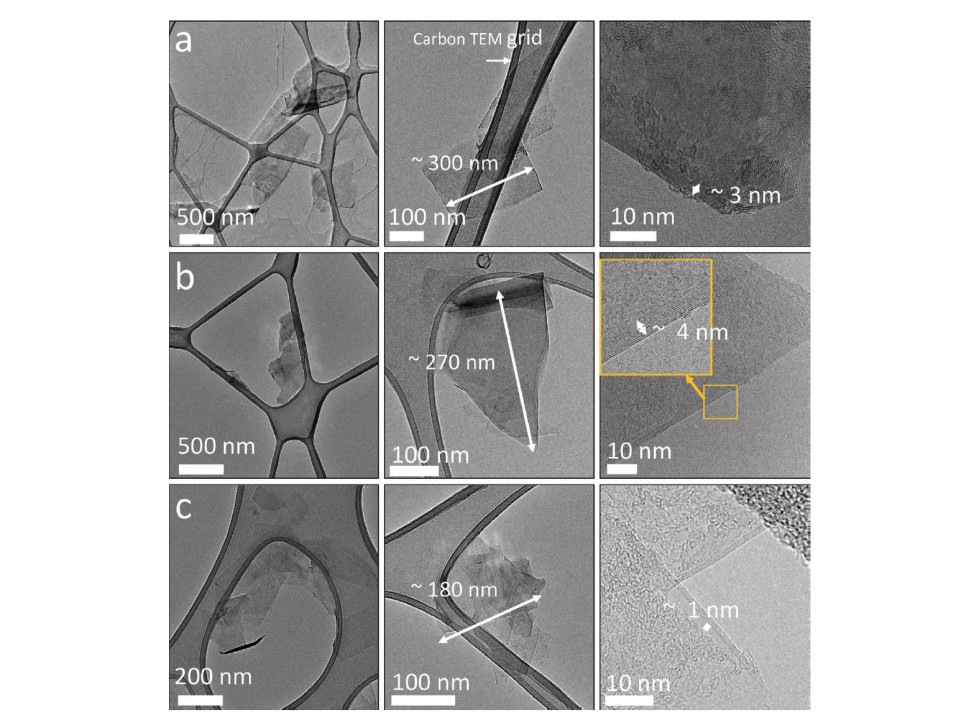
Graphene, a remarkable allotrope of carbon, has recently garnered immense attention due to its extraordinary properties, balancing both density and flexibility in an unprecedented manner. Understanding why graphene possesses these contrasting characteristics not only sheds light on its potential applications but also demonstrates fundamental principles of material science and condensed matter physics.
At the atomic scale, graphene is simply a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice. This seemingly simple structure belies its complexity. Each carbon atom in graphene forms three covalent bonds with its neighbors, resulting in a planar configuration. The strength of these bonds contributes significantly to graphene’s high tensile strength, which measures approximately 130 gigapascals—over 100 times stronger than steel. However, underneath this rugged surface is a fascinating interplay of atomic structure and bonding that explains graphene’s unique capabilities.
One critical aspect that renders graphene both dense and flexible lies within its carbon-carbon bond length. The bonds in graphene are characterized by sp2 hybridization. These hybridized bonds create a robust lattice that facilitates electron delocalization. As a result, graphene exhibits exceptional mechanical properties, such as elasticity and flexibility. The short bond lengths inherently foster tight packing of atoms, granting graphene its impressive density while simultaneously allowing for deformation without rupture.
Moreover, the atomic structure of graphene promotes the phenomenon known as the Dirac cone and massless fermions. In this context, electrons behave as if they are massless particles, enabling unprecedented mobility, which is integral for flexibility. When subjected to external forces, the bonds can stretch and compress, allowing the structure to absorb energy without permanent damage. It is this delicate balance of elasticity governed by the underlying quantum mechanical principles that contributes to the material’s dual nature.
The density of graphene stems not only from its atomic arrangement but also from the nature of carbon itself. Carbon atoms are tightly packed in the graphene lattice, allowing for a high surface area-to-volume ratio which enhances its density metrics significantly. Yet, despite this density, graphene maintains a lightweight profile that is nearly weightless—another wonderful paradox that emphasizes its strategic importance in applications ranging from electronics to material engineering.
An interesting corollary to graphene’s properties is the capacity for functionalization. The inherent flexibility of graphene allows for different chemical modifications, whereby various functional groups can be attached to its surface without compromising its structural integrity. This functionalization opens avenues for tailoring its mechanical properties. For instance, altering the surface characteristics can enhance bonding interactions, improving the performance of composites or coatings, thus preserving graphene’s density while also augmenting its flexibility in varied environments.
The underpinning reasons for the fascinating attributes of graphene extend beyond its atomic structure into the realm of phonon behavior. Phonons, the quantized units of lattice vibrations, play a notable role in thermal conductivity and sound propagation through materials. In graphene, the peculiar interaction between electrons and phonons facilitates remarkable thermal transport properties. Essentially, the flexibility of the carbon bonds permits deformation modes that minimize thermal resistance while preserving substantial energy density. This understanding emphasizes the material’s capability not only to endure stress but also to maintain structural integrity across various temperatures and conditions.
The adaptability of graphene is also visible in its capability to form composite materials. When combined with polymers, metals, or ceramics, graphene can confer strength and flexibility, enhancing overall performance metrics, such as fatigue resistance and thermal stability. The synergy between graphene’s rigidity and various other materials’ properties not only results in composites that are lighter but also tremendously more durable, opening pathways for innovative engineering solutions.
Graphene’s applications illustrate the profound impact of its unique combination of density and flexibility on modern technology. In the fields of electronics, graphene is lauded for its potential to revolutionize transistors, sensors, and flexible touchscreens. Its high electron mobility ensures rapid signal transmission, while its flexibility allows for innovative form factors previously relegated to aspirations. Such devices can bend and flex without losing functionality, presenting exciting prospects in consumer gadgets and wearables.
Furthermore, in energy storage technologies, graphene plays a pivotal role. Utilizing its surface area and density conducive to capacitance, graphene-based supercapacitors and batteries can achieve higher energy storage and faster charge times compared to conventional materials. This expands its use not only in electronics but also in renewable energy applications, contributing to more efficient solar cells, fuel cells, and power systems.
In conclusion, the dual characteristics of density and flexibility in graphene can be appreciated as a product of its atomic structure, bonding dynamics, and phonon behavior. The remarkable ability of graphene to maintain integrity under mechanical deformation while exhibiting high density arises from a delicate interplay of quantum mechanics and chemical bonding. This acknowledged balance has positioned graphene at the forefront of materials science, illuminating new possibilities across diverse technological applications. As research evolves, the fascination surrounding graphene will likely continue to yield insights that blur the boundaries between science fiction and scientific reality.