Graphene oxide (GO) has emerged as a tantalizing subject in materials science due to its unique properties and potential applications across various fields. However, the reduction of graphene oxide is a nuanced process that raises a pivotal question: why do we reduce graphene oxide in the first place? This inquiry not only leads us to explore the underlying chemistry but also invites us to engage with the myriad implications of reduced graphene oxide (rGO) in practical applications. The journey from graphene oxide to its reduced counterpart presents a fascinating interplay of challenges and opportunities that deserve thorough examination.
The primary motivation behind reducing graphene oxide lies in its intrinsic electronic properties. Graphene oxide is characterized by its rich functional group content, primarily oxygen functionalities such as hydroxyl, epoxy, and carbonyl groups. While these functional groups impart significant solubility and processability to GO, they simultaneously compromise its electrical conductivity. Transitioning from GO to rGO effectively facilitates the restoration of the delocalized π-electron system found in pristine graphene, optimizing its conductivity. As such, one of the foremost reasons for reduction is to enhance the electrical characteristics necessary for applications in sensors, transistors, and energy storage devices.
Moreover, reducing graphene oxide enhances its mechanical properties. Graphene, in its pure form, exhibits exceptional tensile strength, which is significantly diminished in its oxidized state. Upon reduction, the restoration of sp2 hybridization leads to improvements in both strength and flexibility. Researchers investigating the composite materials often seek to exploit this enhanced mechanical prowess, integrating rGO into polymers, ceramics, and metals to create materials that surpass conventional limits in strength and weight.
The reduction process, however, is not devoid of complexities. It prompts an essential inquiry into the methods available for achieving a successful reduction of GO. Various chemical, thermal, and electrochemical reduction techniques abound, each with distinct advantages and potential pitfalls. Chemical reduction, for instance, often involves strong reducing agents like hydrazine or sodium borohydride. Although effective, these methods can introduce impurities that may alter the surface chemistry and hinder the functionality of the resulting rGO.
On the other hand, thermal reduction represents a promising avenue, where elevated temperatures facilitate the removal of oxygen functionalities. While this method can yield high-quality rGO, it is limited by scalability and the energy costs associated with high-temperature processing. Another innovative approach is electrochemical reduction, which allows for the tuning of reduction levels via controlled charging conditions. This dynamic technique presents a fascinating landscape for material scientists, posing the question: how can one optimize the reduction conditions to yield rGO with desired properties?
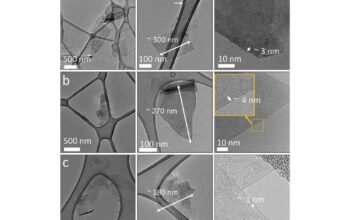
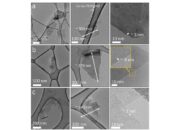
Once synthesized, rGO presents a unique set of challenges in terms of characterization. The reduction process often leads to heterogeneous materials, where the degree of reduction may vary across the sample. Consequently, establishing a unified metric for evaluating the quality of rGO becomes a formidable task. Techniques such as Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and atomic force microscopy (AFM) are indispensable for providing insights into the structural and electronic attributes of reduced materials. Employing these methodologies effectively requires a deep understanding of their implications in assessing material properties.
Furthermore, the resultant rGO interacts with its environment in fascinating ways, which can be an advantage or a limitation depending on the application. The presence of residual functional groups post-reduction can influence rGO’s interactions with other materials, often enhancing its compatibility in composites. While this is advantageous in certain scenarios, such as in biomedical applications where cell interaction is critical, it may not be ideal for applications requiring high purity and conductivity. Understanding how these post-reduction characteristics affect performance is imperative.
Considering the energy landscape, the integration of rGO in energy storage and conversion technologies represents one of the most compelling applications. As researchers strive to improve energy density and cycling stability in batteries and supercapacitors, rGO emerges as a crucial component. The question arises: how does the reduction process affect the electrochemical performance of these devices? Studies suggest that the specific surface area and conductivity are pivotal for enhancing capacitance and charge-discharge rates, illustrating the importance of meticulous reduction methodologies.
Moreover, in the field of sensors, rGO’s high surface area and unique properties enable it to detect minute concentrations of gases and biomolecules. This is particularly noteworthy in environmental monitoring and medical diagnostics. However, it also raises the challenge of reproducibility; ensuring consistent sensor performance requires a deep understanding of the reduction parameters and their influence on surface chemistry.
To encapsulate, the reduction of graphene oxide is significantly more than a mere chemical transformation; it is a complex journey that intertwines scientific innovation with practical challenges. The fascinating transition from GO to rGO underscores a vital narrative in materials science, offering boundless opportunities while simultaneously demanding rigorous research and development. With myriad applications on the horizon, one must ponder how we can overcome the existing challenges in the reduction process, paving the way for a new era of advanced materials that can reshape industries.