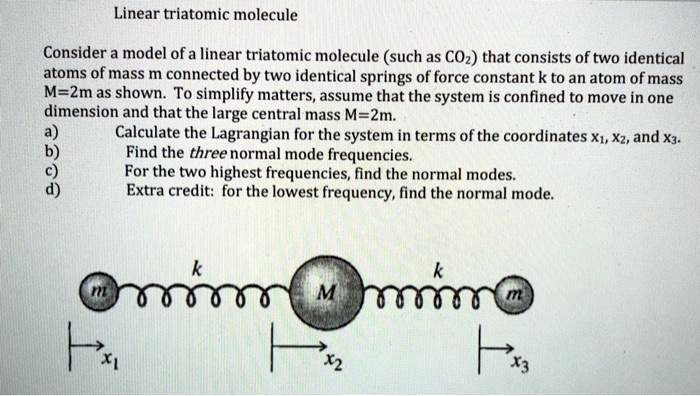
In the realm of molecular chemistry, the classification of molecules based on their geometry is essential in understanding their physical properties and chemical behavior. Among these, triatomic molecules, which consist of three atoms, can exhibit various shapes. One particularly intriguing configuration is the linear formation. This article delves into the unique characteristics of linear triatomic molecules, examining the underlying principles of their geometry, the types of bonds involved, and the implications for their physical and chemical properties.
1. Definition and Characteristics of Triatomic Molecules
A triatomic molecule is defined as a molecular entity comprising three atoms, which may be identical or different. The linear configuration is achieved when these three atoms are arranged in a straight line, with bond angles measuring 180 degrees. Notably, linear triatomic molecules demonstrate specific traits that distinguish them from their non-linear counterparts. Such features include symmetry, vibrational modes, and bond lengths.
2. Geometric Arrangements and Electrons
The geometry of a molecule is primarily dictated by the valence shell electron pair repulsion (VSEPR) theory. This theory posits that electron pairs surrounding a central atom arrange themselves to minimize repulsion, leading to distinct geometrical shapes. In a linear triatomic molecule, the central atom is flanked by two other atoms, creating a simplified system where the bond angles are maintained at a straight line. The stability of this configuration arises from the optimal spatial arrangement of electron density around the central atom, further reinforced by the types of chemical bonding involved.
3. Types of Bonds in Linear Triatomic Molecules
Linear triatomic molecules can involve various types of bonding: covalent, ionic, or metallic. Covalent bonding, characterized by the sharing of electron pairs between atoms, is particularly prevalent among linear triatomics. Consider carbon dioxide (CO2) as a quintessential example; it consists of one carbon atom covalently bonded to two oxygen atoms. This molecular configuration exhibits two double bonds, contributing to its linear geometry.
Another aspect to consider is the molecular hybridization that influences bonding. In CO2, the carbon atom undergoes sp hybridization, facilitating the formation of two equivalent sp hybrid orbitals. These orbitals align linearly with the oxygen atoms, resulting in a symmetrical distribution of electrons. In contrast, when examining ionic compounds composed of triatomic molecules, such as the linear carbonate ion (CO32-), a different bonding dynamic is present, wherein ionic interactions manifest between charged entities rather than sharing electrons freely.
4. Symmetry and Physical Properties
The linear arrangement of triatomic molecules results not only in aesthetic uniformity but also in symmetric properties that extend to their physical behaviors. The symmetrical nature of linear molecules often gives rise to distinct spectroscopic characteristics, which can be invaluable in identifying molecular species. For instance, vibrational spectra are significantly impacted by molecular geometry. Linear triatomic molecules may display simpler vibrational modes due to the restricted movement, while non-linear configurations often lead to additional vibrational modes, complicating their spectra.
Moreover, the linear geometry plays a crucial role in determining the thermodynamic properties of triatomic molecules. A classic instance is the effect on boiling and melting points; the symmetrical distribution of charge in linear molecules can translate to lower polarizability compared to their non-linear counterparts. Consequently, such factors can significantly influence the state of a given compound under varied temperature conditions.
5. Applications and Implications
Understanding the linear nature of triatomic molecules is paramount in numerous scientific disciplines. In atmospheric science, for instance, CO2 is acknowledged as a greenhouse gas with linear attributes that allow it to absorb infrared radiation—a crucial factor in climate modeling and environmental studies. The linear configuration contributes to its effectiveness in trapping heat, further emphasizing the importance of geometry in molecular interactions.
Additionally, in the realm of materials science, linear triatomic molecular structures can be exploited in the design of polymers and nanomaterials. The inherent linearity may impart desirable mechanical properties, such as tensile strength and flexibility, which are vital in applications ranging from industrial materials to biomedical devices.
6. Conclusion
In summation, the linear arrangement of triatomic molecules is a testament to the intricate interplay of chemical bonding, electron configuration, and molecular geometry. Through a combination of covalent or ionic bonding, effective electron repulsion management, and symmetry, these molecules exhibit unique physical and chemical properties that are pivotal in various scientific applications. As research into molecular structures continues, the exploration of linear triatomic molecules remains a key area of interest, with implications that stretch across chemistry, environmental science, and material innovation.