Graphite, a crystalline form of carbon, exhibits a unique layered structure that has fascinated scientists and engineers alike. This structural arrangement not only contributes to the physical properties of graphite but also enables a plethora of applications ranging from lubricants to batteries. Understanding the underlying reasons for graphite’s layered architecture requires an exploration of its atomic structure, bonding characteristics, and the implications of those properties in various contexts.
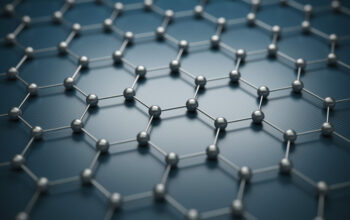
At the atomic level, graphite is composed of carbon atoms arranged in a two-dimensional honeycomb lattice. Each carbon atom covalently bonds to three other carbon atoms through sp2 hybridization. This bonding configuration engenders a planar structure where carbon atoms form hexagonal rings. The fourth valence electron of each carbon atom remains in a p-orbital, which is not involved in the bonding but contributes to a delocalized π-electron cloud above and below the carbon layers. This delocalization is significant as it results in enhanced electronic conductivity, making graphite an excellent conductor of electricity.
The individual planes of carbon atoms within graphite are held together by weak van der Waals forces. These interplanar forces are significantly weaker than the covalent bonds within the planes, allowing the layers to slide over one another with relative ease. This characteristic gives graphite its lubricating properties, making it suitable for applications in various industrial processes. The layered structure also results in significant anisotropy in physical properties; for instance, the thermal and electrical conductivities are much higher parallel to the layers compared to perpendicular to them.
Moreover, the layered architecture of graphite can be attributed to its stability and formation energy. Thermodynamically, the layered structure represents a state of lower energy compared to a three-dimensional bulk arrangement of carbon atoms. In conditions of high temperature and pressure, carbon tends to favor the formation of diamond, which is a three-dimensional network of carbon atoms. However, at ambient conditions, the stability of the layered structure of graphite is thermodynamically favored. This preference stems from the balance between the energy contributed by strong covalent bonding within the layers and the weaker van der Waals interactions between the layers.
Graphite’s layered nature also provides insights into various phenomena such as intercalation, where foreign atoms or molecules can enter the spaces between the layers. This property is leveraged in applications such as lithium-ion batteries, where lithium ions can be intercalated into graphite anodes during charge and discharge cycles. The ability to accommodate these ions without significant structural deformation is a direct consequence of its layered configuration. This intercalation not only enhances the electrochemical performance but also serves to further enrich the diverse applications of graphite.
Furthermore, the anisotropic characteristics of graphite have profound implications in the field of materials science. Researchers are exploring methods to exploit these properties to create composite materials that optimize the mechanical strengths of aligned graphite fibers while maintaining the lightweight benefits typical of carbon materials. Such composites can be instrumental in aerospace and automotive applications, where weight reduction and enhanced material strength are critical considerations.
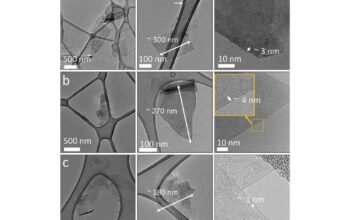
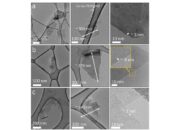
Another fascinating perspective on graphite’s layered structure involves its interaction with various chemical species. The layers of graphite can interact with polar and non-polar molecules differently. This behavior makes graphite an essential material in catalysts and adsorbents. The active sites located at the edges or defects in the layers can facilitate chemical reactions, adding another layer of functionality to this already versatile material. Furthermore, the surface chemistry of graphite can be modified through oxidation, resulting in materials like graphene oxide, which exhibit distinct properties due to the introduction of functional groups that increase hydrophilicity.
The exploration of alternative allotropes of carbon such as graphene and carbon nanotubes is, in many ways, an extension of the examination of graphite’s layered structure. Graphene, which is a single layer of carbon atoms arranged in a two-dimensional lattice, inherits many of the unique properties of graphite, such as high thermal and electrical conductivity, but exhibits even greater strength and flexibility. This relationship illustrates a continuum of structural sophistication in carbon allotropes, further underlining the significance of graphite as a foundational material in nanotechnology and advanced material design.
In summary, the reason behind graphite’s layered structure is a multi-faceted phenomenon that incorporates its distinctive bonding characteristics and thermodynamic stability. The interaction of covalent bonding within the layers and van der Waals forces between them not only defines the lattice structure but also opens up avenues for a myriad of applications. This duality of stability and functionality highlights an intricacy that is often overshadowed by graphite’s everyday applications. As research progresses, the layered architecture of graphite continues to inspire innovations across various scientific and engineering domains, reinforcing the idea that the most fundamental structures often possess the most profound implications for technological advancement.