Graphene, a monolayer of carbon atoms arranged in a two-dimensional lattice, has garnered immense attention in the scientific community for its exceptional properties, including high electrical conductivity, remarkable mechanical strength, and excellent thermal conductivity. With such a potent combination of attributes, the allure of synthesizing graphene at home captures both the imagination of amateur scientists and the ambitions of experienced researchers alike. Understanding the most effective methods for graphene production not only highlights the advances in material science but also unveils the potential for innovations in various applications, ranging from electronics to biomedicine.
Before discussing methodologies for home synthesis, it is imperative to appreciate the underlying reasons for the fascination with graphene. Its atomic structure lends it unique characteristics that are not only visually striking but also functionally consequential. This duality of aesthetic and utility propels interest in creating this remarkable material, whether for personal experimentation or broader scientific inquiry.
Among the various methodologies for synthesizing graphene, two prominent techniques have surfaced as accessible for home experimentation: mechanical exfoliation and chemical reduction of graphene oxide (rGO). Each method presents its own distinct pathway, and understanding their mechanics is pivotal for any experimental endeavor.
Mechanical Exfoliation
Mechanical exfoliation, often referred to as the “Scotch tape method,” represents one of the simplest and most effective methods for obtaining high-quality graphene. This technique involves the systematic peeling away of graphite layers using adhesive tape.
The procedure begins with high-purity graphite, readily available in various forms, such as pencil lead or graphite powder. Strong adhesive tape is used to wrap around the graphite, after which the tape is repeatedly folded and unfolded. This action successfully detaches thin layers of graphite, ultimately leading to the formation of monolayers and bilayers of graphene.
While this method is straightforward, it boasts significant limitations concerning scalability. The resultant sheets may be irregular in size and thickness, and the yield is often inconsistent. Nevertheless, the satisfaction of producing visible sheets of graphene using this rudimentary approach is a compelling reason for many to pursue the mechanical exfoliation method.
Chemical Reduction of Graphene Oxide
In contrast, chemical reduction of graphene oxide provides a more scalable approach, albeit with some compromises in quality compared to pristine graphene. This method involves the oxidation of graphite to produce graphite oxide, which is then reduced, typically via a chemical agent, to obtain graphene oxide (GO), ultimately yielding reduced graphene oxide (rGO).
The first stage of the process requires treating graphite with strong oxidizing agents—commonly a mixture of sulfuric acid, phosphoric acid, and potassium permanganate in a controlled environment. The oxidation breaks down the graphite into multilayered graphene oxide, which possesses hydroxyl and epoxy groups. These functional groups play a crucial role in enhancing the solubility of graphene oxide in water, thus facilitating further processing.
Following oxidation, the reduction phase can be initiated through various methods, including thermal treatment, chemical reduction with hydrazine, or electrochemical processes. Each method carries its own advantages and drawbacks. For instance, thermal reduction may yield relatively high-quality rGO, while chemical reduction provides faster yields.
An important consideration in the reduction process is the extent to which functional groups are removed. The presence of residual oxygen-containing groups can significantly impact electronic properties and overall performance. Achieving a balance between reduction and retention of desirable functionalities is essential for optimizing the outcome.
Post-Synthesis Characterization
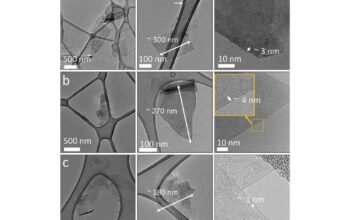
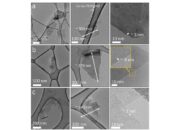
Regardless of the synthesis method employed, comprehensive characterization of the resulting graphene material is paramount. Techniques such as Atomic Force Microscopy (AFM), Scanning Electron Microscopy (SEM), and Raman Spectroscopy serve as vital tools in determining the quality and purity of the synthesized graphene.
AFM provides high-resolution topographical maps, revealing the thickness and overall morphology. SEM allows visualization of morphology and surface features at a nanoscale level, uncovering details of the layered structure. Raman Spectroscopy offers insights into the structural integrity of graphene through defect density analysis, typically represented by the intensity ratios of the D and G bands, denoting defect-related and graphitic features respectively.
Applications and Future Prospects
The implications of home-synthesized graphene extend far beyond the confines of personal experimentation. Graphene’s versatility opens avenues for application in various domains, including but not limited to energy storage, flexible electronics, bio-sensing, and composite materials. The demand for sustainable graphene production has spurred ongoing research into eco-friendlier synthesis methods.
As enthusiasts and professionals alike venture into home synthesis, the convergence of curiosity and scientific rigor fosters an environment ripe for innovation. From enhancing conductivity in consumer electronics to developing new drug delivery systems, the potential applications of graphene synthesized at home underscore the material’s transformative capabilities.
In summary, synthesizing graphene at home, through either mechanical exfoliation or the chemical reduction of graphene oxide, offers a gateway into the exciting world of material science. The fascination surrounding graphene, rooted in its unparalleled properties, drives individuals to explore its synthesis and application. By overcoming challenges associated with quality and scalability, home experimentation can contribute to a more profound understanding and utilization of this extraordinary material, revealing a landscape rich with scientific and innovative promise.