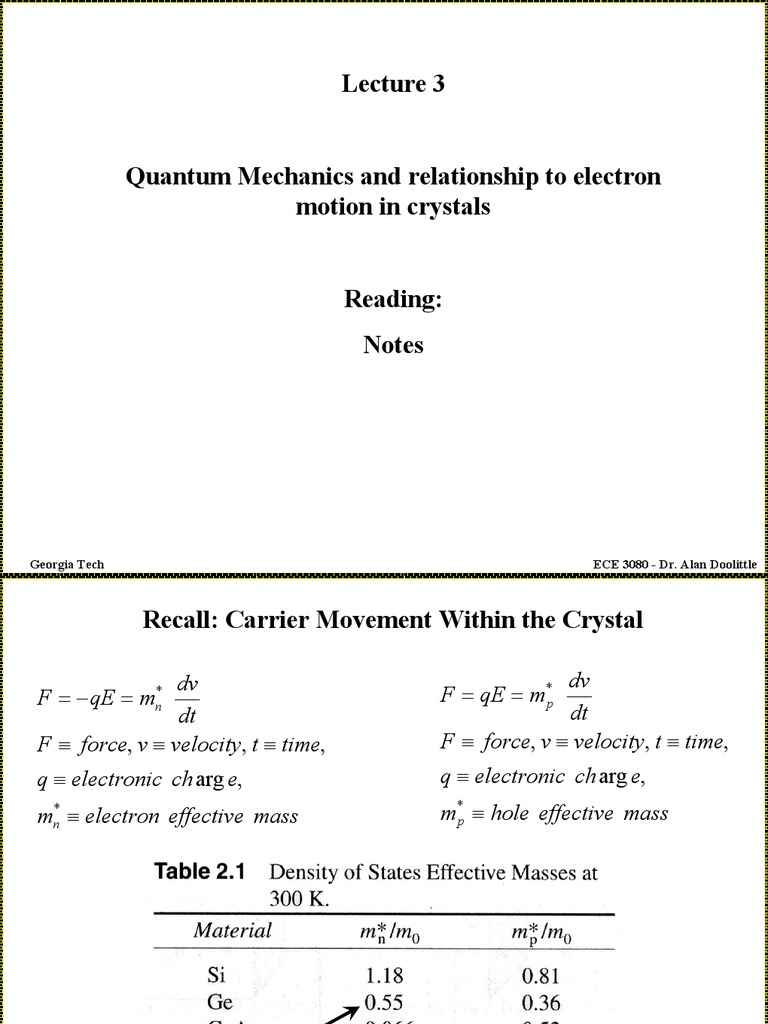
Quantum chemistry emerges as a sublime confluence of classical chemistry and quantum mechanics, offering insights that transcend conventional paradigms. As Schrödinger meets the molecule, a rich tapestry of interactions unfolds, revealing the intricate dance of electrons and nuclei that defines chemical behavior at the most fundamental level. This exploration unveils the mysteries of matter, adeptly traveling through the interstices of scientific discipline and philosophy, urging us to ponder the very nature of reality.
The roots of quantum chemistry sprout from the necessity to explain phenomena that classical chemistry could not address adequately. The early 20th century heralded a revolution, wherein quantum mechanics redefined our understanding of atomic and molecular systems. The Schrödinger equation, a mathematical embodiment of the quantum world, serves as the cornerstone of this discipline. By articulating the wave function of a system, it encapsulates the probabilistic nature of particles, thus enabling chemists to predict the behavior and characteristics of molecules.
To comprehend quantum chemistry, one must first grapple with the duality inherent in quantum mechanics. Particles exhibit both wave-like and particle-like properties, culminating in a bewildering yet captivating phenomenon. This duality is analogous to a river that flows with both fluidity and structure, capable of shaping landscapes while simultaneously carving its own path. In quantum chemistry, the wave function of an electron not only describes its location but also embodies its energy levels and interactions with other particles, evoking a rich interplay akin to a grand symphony wherein each instrument contributes to a harmonious whole.
At the heart of quantum chemistry lies the concept of electron configuration, a foundational element that delineates how electrons occupy the available atomic orbitals. The principles of quantum mechanics dictate that electrons inhabit discrete energy levels, akin to performers confined to specific stage sections, yet able to orchestrate complex interplays when they leap between levels. This quantization introduces stability in atomic structure, for no two electrons may occupy the same quantum state simultaneously. The Pauli exclusion principle thus reigns supreme, ensuring that amidst the chaos, a delicate equilibrium is maintained.
Utilizing quantum mechanics, chemists have unearthed the intricate bonding patterns that undergird molecular formation. Covalent bonds, characterized by shared electrons, can be envisioned as collaborative partnerships among atoms, each negotiating its role to achieve stability. This bonding generates a tapestry of molecular geometry, which is pivotal in defining the physical properties of compounds. Moreover, quantum chemistry elucidates the concept of molecular orbitals—constructs that result from the linear combination of atomic orbitals. This amalgamation reveals how electron density is distributed across a molecule, adding layers of complexity to our understanding of chemical reactivity and interaction.
Additionally, quantum chemistry unveils the subtle forces that govern molecular interactions. The phenomenon of resonance—the ability of a molecule to exist in multiple forms—exemplifies the elegant dilemma of chemical stability versus energetic favorability. It is here that the concept of potential energy surfaces comes into play, charting the energetic landscape that a system traverses during its chemical transformations. Thus, when molecules engage in reaction pathways, they navigate this topographical map, often encountering energy barriers that can be likened to mountains on a graph of molecular potential.
The utilization of quantum chemistry in computational methods has revolutionized the approach to molecular design and analysis. Techniques such as Density Functional Theory (DFT) and Hartree-Fock calculations allow chemists to predict properties with remarkable accuracy, permitting the simulation of molecular behavior under various conditions. By adopting this computational framework, scientists can probe into the realms of materials science, drug discovery, and catalysis. In these pursuits, quantum chemistry not only aids in the rational design of new compounds but also enhances our comprehension of existing substances, fostering innovation in various fields.
A burgeoning area within quantum chemistry is the interplay between quantum mechanics and thermodynamics, particularly the study of quantum entropy and its implications for molecular systems. This fusion delves into the complexities surrounding enthalpic and entropic contributions to reactions, shaping our understanding of spontaneity in chemical processes. As one enumerates the contributions to free energy, it becomes evident that the molecular world is governed by more than mere reactions; it is an evolving tapestry where statistics and probabilities dictate pathways toward equilibrium.
Moreover, the philosophical implications of quantum chemistry invite contemplation on the nature of reality itself. The notions of superposition and entanglement beckon towards existential inquiries, suggesting that the universe manifests in states far removed from a deterministic cause-and-effect paradigm. In embracing this quantum perspective, we transition from a rigid understanding of molecules as static entities to a more dynamic outlook where interactions and probabilities reign supreme. This shift stimulates profound thoughts on everything from consciousness to environmental sustainability, revealing the expansive implications of quantum mechanics beyond the confines of the laboratory.
In conclusion, quantum chemistry stands at the intersection of art and science, where Schrödinger’s insights intersect with the complexity of molecular systems. It invites us to explore the unseen forces orchestrating the elemental dance of matter and energy. Through a profound understanding of the quantum realm, we gain access to the keys of innovation, unlocking new vistas of knowledge that promise to reshape our world. Each equation, each reaction, illuminates new pathways, much like a starlit sky revealing constellations of thought, navigating the profound depths of the molecular universe.