In the grand theater of atomic structure, electrons hold a crucial yet oft-misunderstood role, serving as the flickering actors on the stage of matter. Much like dancers who bring to life the vibrancy of a performance, electrons dictate the intricate behaviors and properties of atoms. To delve into which electrons are responsible for these qualities, one must embark on a journey through the layered complexity of atomic architecture, where each electron’s role is critical to the overall functionality of the atom.
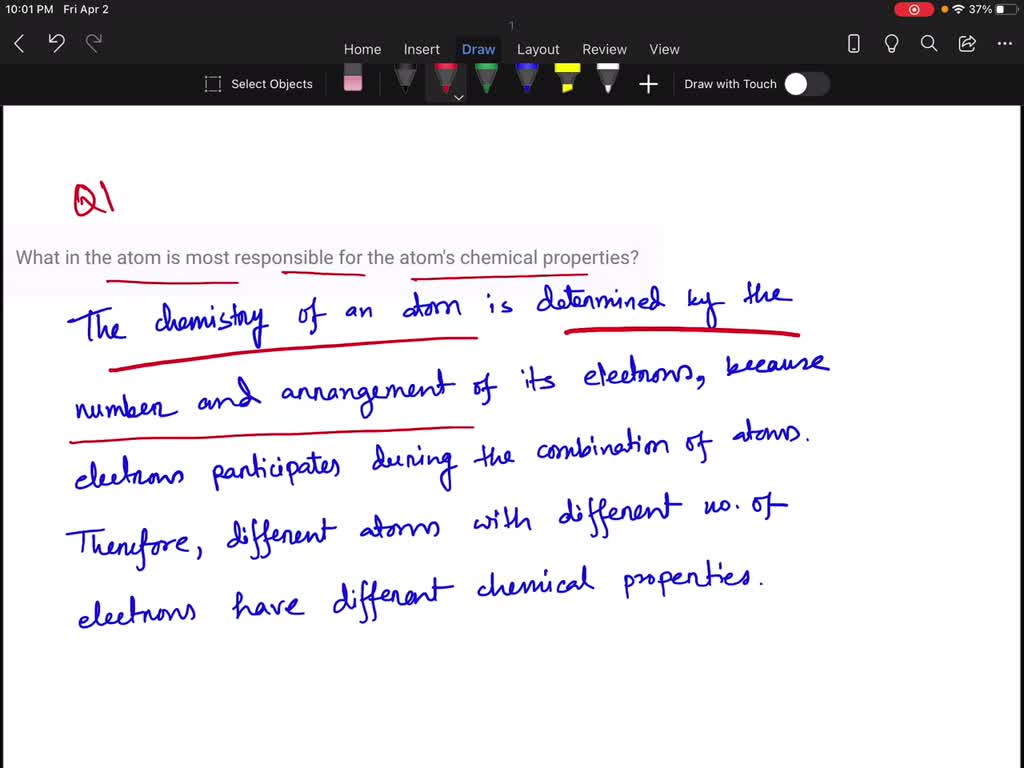
The atom, the fundamental building block of all matter, is composed of a nucleus flanked by a cloud of electrons. At the heart of the atom resides the nucleus, a dense region consisting of protons and neutrons. However, it is the electrons that twirl and swirl around this nucleus, engaging in interpersonal interactions, that ultimately dictate the physical and chemical properties of the atom. The most vital players in this grand ballet are the valence electrons.
Valence electrons reside in the outermost shell of an atom’s electron cloud. This is akin to the outermost layer of an onion, where the essential flavors reside. Their energy levels are higher than those of the core electrons, granting them a unique capacity to interact with other atoms. In the pursuit of stability, these electrons will either bond, donate, or accept electrical charges. Thus, the valence electrons become the architects underlying chemical bonds, significantly influencing the reactivity and properties of different elements.
For instance, consider the reactivity of alkali metals such as sodium. With a single valence electron, sodium is eager to shed this electron, embarking on a quest for stability by forming ionic bonds with other elements. The simplicity of obtaining a stable electron configuration transforms sodium from merely a reactive metal to a fundamental building block of various compounds, including table salt. The valence electron’s role can be likened to a key that unlocks the myriad of interatomic interactions, revealing vibrant chemical properties.
While valence electrons predominantly determine an atom’s chemical behavior, core electrons also merit attention. Although they reside deeper within the atomic structure, core electrons provide a shielding effect for valence electrons. This is akin to a protective barrier that moderates the interaction between an atom and its surrounding environment. Core electrons are involved in the stabilization of the electron cloud, reassuring valence electrons as they partake in their energetic dances across chemical landscapes.
The nuances of electron configuration further illuminate the unique roles of specific electrons in determining atomic properties. For example, the arrangement of electrons in an atom follows the principles set forth by quantum mechanics. The Pauli Exclusion Principle and Hund’s Rule manifest in the filling of atomic orbitals, dictating how electrons populate various energy levels. This configuration not only influences atomic size and electronegativity but also contributes to the unique identities of elements. The introduction of noble gases into the discussion—a group characterized by complete electron shells—highlights how full outer orbital configurations render these elements less reactive, displaying remarkable inertness.
Spectroscopic techniques offer a captivating glimpse into the electron world, revealing how transitions between energy levels yield information about an atom’s properties. When an electron absorbs energy, it may transition to a higher energy state, only to relax—releasing energy in the form of light upon returning to its original configuration. This phenomenon is the foundation behind the colorful emissions of fireworks and the hues seen in spectral lines, vividly linking electron behavior to observable characteristics of matter.
Another fascinating context in which electrons assume significance is the realm of metals and their conductivity. The sea of delocalized electrons present in metallic structures creates a unique scenario where these electrons can freely move, imparting metals with their characteristic electrical conductivity. The attraction among positively charged metal ions and these mobile electrons bestows unique properties such as malleability and ductility, allowing metals to be shaped without breaking. In this respect, it is the behavior of these valence electrons that confers upon metals their metallic luster and ability to conduct electricity, showcasing their essential role in the larger picture of material science.
The study of chemistry and physics often converges as one explores the implications of electron behavior in magnetism. Unpaired electrons within an atom contribute to its magnetic properties, where their orientations dictate whether the material behaves as a magnet or not. In simple terms, when the magnetic moments of unpaired electrons align in the same direction, the material exhibits ferromagnetism. Hence, beyond bonds and reactivity, the role of electrons expands into the captivating domain of magnetism—a radiant property that exemplifies the interplay between quantum mechanics and classical phenomena.
To summarize, electrons, particularly valence electrons, are the unsung heroes of atomic properties. They dictate the chemical behaviors, the interactions with various environments, and the very essence of material characteristics. Just as artists enliven a canvas, electrons breathe life into the fabric of matter, shaping everything from the simplest compounds to intricate biological structures. Through their intricate dance, electrons carve out the landscape of the universe—humble yet powerful, frenetic yet serene, they embody the essence of atomic existence. The next time one gazes upon a substance, pondering its properties, remember that the secrets lie within its electrons, ever residing in their ethereal ballet, ever defining the nature of reality itself.