Graphene, an allotrope of carbon, has gained immense attention in the realms of materials science, chemistry, and physics due to its extraordinary properties that encompass high electrical conductivity, remarkable mechanical strength, and exceptional thermal properties. The aspiration to harness graphene’s potential has spurred research into cost-effective synthesis methods. This article elucidates various inexpensive avenues for creating graphene, examining techniques that manipulate carbon structures while considering scalability and environmental implications.
To comprehend the methods for cheap graphene production, it is imperative to first acknowledge the diverse forms in which graphene can manifest. Conventional routes involve techniques such as chemical vapor deposition (CVD), liquid-phase exfoliation, and chemical reduction of graphene oxide. Nevertheless, each method’s financial implications can vary significantly, leading to the pursuit of more economical alternatives.
One of the most straightforward and prevalent approaches for synthesizing graphene involves mechanical exfoliation, often referred to as the “Scotch tape method.” This technique exploits the innate properties of graphite. By applying adhesive tape to a piece of graphite, the tape can peel off layers, yielding thin sheets of graphene. While this method is exceptionally simple and low-cost, it is time-consuming and can produce limited quantities of material. Yet, for educational purposes or small-scale applications, this technique remains a viable choice.
To enhance production efficiency while maintaining a low budget, the liquid-phase exfoliation method offers a compelling alternative. This process involves sonication in solvents, where graphite flakes are dispersed in a liquid medium followed by ultrasound treatment. The vigorous agitation generates shear forces that effectively detach graphene layers from the bulk graphite. While this method can be performed using relatively inexpensive household sonication equipment, the quality and uniformity of the produced graphene may vary based on the choice of solvent and graphite quality. Additionally, developing scalable processes to facilitate larger volumes remains a challenge.
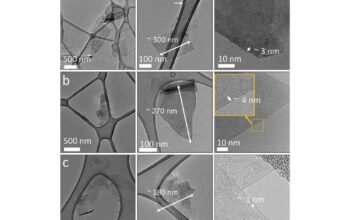
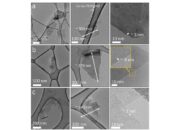
Another economical approach involves the chemical reduction of graphene oxide (GO), a precursor in the production of graphene. The process begins with the oxidation of graphite, utilizing reagents such as potassium permanganate and sulfuric acid, which break down the graphite structure into individual graphene oxide sheets. Subsequently, these oxide sheets can be reduced through mild chemical agents like hydrazine or even more benign alternatives such as vitamin C. Although the oxidation process may entail higher initial costs, the reduction step significantly augments the yield of graphene with favorable electrical properties at relatively modest expenses.
For those keen on innovating within the scope of waste materials, biomass-derived graphene synthesis has emerged as a burgeoning field. Biomass, which encompasses organic material such as agricultural waste, possesses carbon-rich components amenable to conversion into graphene. Techniques including pyrolysis, which involves thermally decomposing biomass in the absence of oxygen, can yield carbon nanostructures, including graphene. The advantages of this method resonate through its sustainability and cost-effectiveness, as it capitalizes on resources that are otherwise considered waste. The challenge lies in optimizing thermal processes to enhance graphene quality while minimizing the production costs further.
Further exploration leads to a technique known as the “sugar method,” which involves the carbonization of sugar (sucrose) as a precursor for graphene synthesis. By heating sucrose under controlled conditions, carbon structures evolve, eventually transitioning into graphene through a rearrangement of its atomic lattice. This attractive method is economically feasible and has gained traction in laboratories aiming for synthetically simple and sustainable graphene production. The major hurdles include controlling the morphology and ensuring uniformity in the resulting product.
Additionally, electrochemical exfoliation of graphite has surfaced as a promising and cost-effective route for synthesizing graphene. This approach utilizes an electrochemical cell to promote the selective exfoliation of graphene layers from graphite sheets, using various ionic liquids and aqueous electrolytes. As this method does not require extensive setup or high-cost materials, it holds the potential for mass production at a fraction of the cost associated with traditional methods, making it a pivotal technique for future scalability.
It is pertinent to address the environmental and health concerns associated with certain graphene production techniques. Approaches that involve toxic chemicals pose risks that can negate cost benefits if not managed properly. Moreover, sustainability considerations should guide future graphene synthesis pathways to ensure ecological balance and public safety.
In conclusion, the quest for economically viable methods for synthesizing graphene is laden with diverse techniques. From mechanical exfoliation and liquid-phase approaches to biomass and sugar-derived methodologies, the landscape of graphene production is evolving. Each method presents a unique array of benefits and challenges that must be reconciled for broad-scale application. Future advancements will likely hinge upon innovating these existing techniques while prioritizing sustainability and safety, paving the way for graphene’s integration into myriad technological applications. As research continues to unveil novel opportunities and refine current methods, the promise held within this remarkable material remains contingent upon the ability to produce it efficiently and affordably.