At the fundamental level of matter, the universe is constructed from an intricate tapestry of atoms, molecules, compounds, and elements. Understanding these concepts offers profound insights into the nature of matter and the universe, revealing the delicate interrelationships that govern chemical interactions and the myriad forms of substances we encounter daily.
1. Atoms: The Building Blocks of Matter
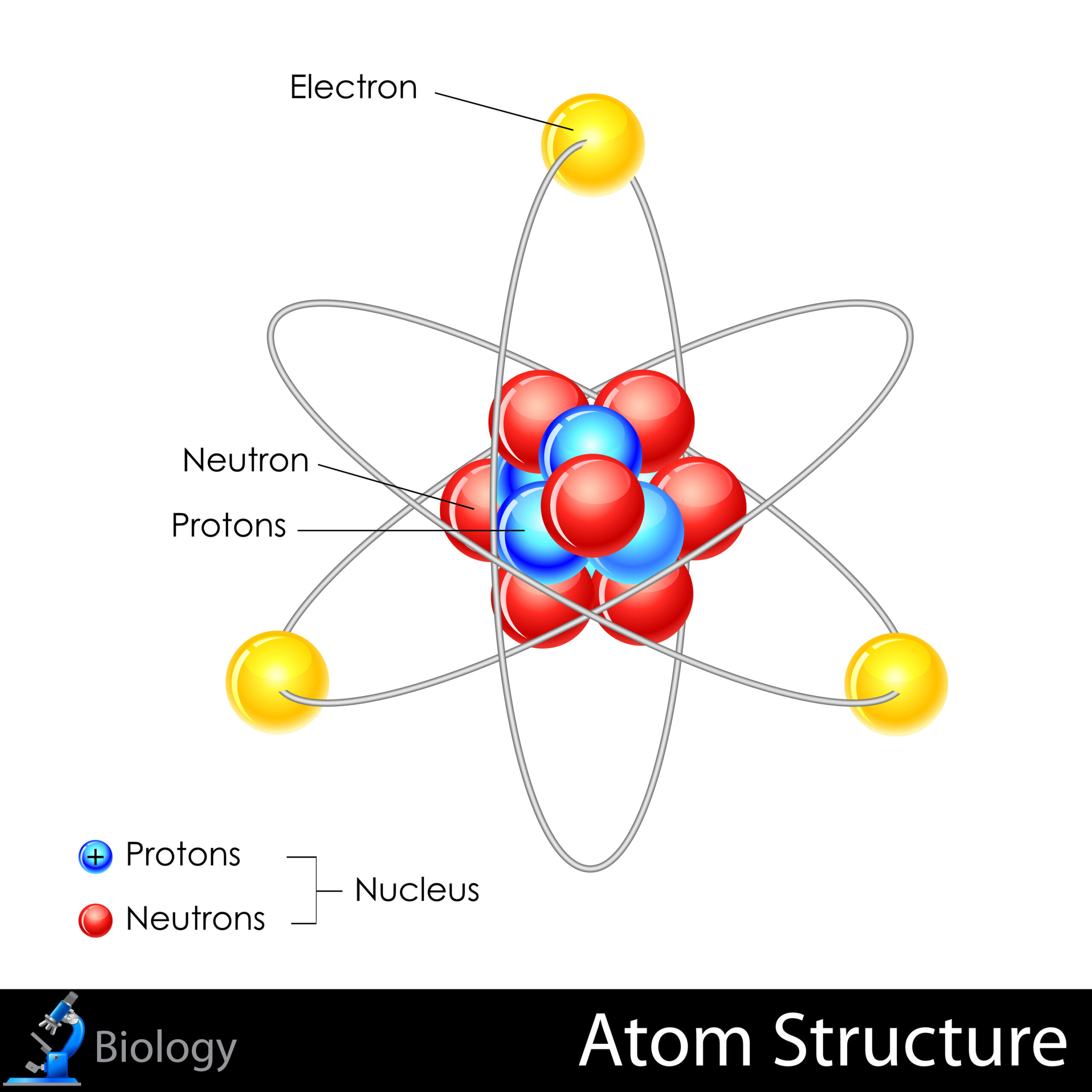
Atoms are the quintessential constituents of all matter. An atom is the smallest unit of an element, retaining the chemical properties of that element. Its structure comprises a nucleus, containing positively charged protons and neutrally charged neutrons, surrounded by a cloud of negatively charged electrons that orbit in various energy levels. This arrangement is central to the atom’s behavior, as the interactions of electrons, particularly the valence electrons in the outer shell, dictate chemical reactivity and bonding characteristics.
The electron configuration within atoms contributes significantly to their stability and interaction potential. For instance, noble gases—elements like helium and neon—display remarkable inertness due to their complete electron shells, enhancing our understanding of stability in atomic structures.
2. Elements: Pure Substances
Elements can be defined as pure substances that cannot be broken down into simpler substances by chemical means. Each element is characterized by its unique number of protons, known as the atomic number. This uniqueness provides the foundational organization of the periodic table. Notably, the periodic table not only categorizes elements based on atomic number but also offers insights into their physical and chemical properties. For example, metals, nonmetals, and metalloids are differentiated by their conductivity, malleability, and reactivity.
Elements manifest various characteristics that reveal their intricate roles in chemical reactions. Oxygen (O), essential for respiration, and carbon (C), the cornerstone of organic chemistry, exhibit vastly different behaviors yet coalesce to form life as we know it. The quest to isolate and understand an element’s properties underscores the essence of chemistry and physics.
3. Compounds: Chemical Combinations
Compounds are substances formed when two or more elements chemically combine in fixed ratios. The interplay between different elements during this process leads to the emergence of novel properties distinct from those of the constituent elements. Water (H2O), composed of hydrogen and oxygen, exemplifies this phenomenon; its properties differ sharply from those of its gaseous elemental forms. The study of compounds illustrates not just the chemical interactions but also sheds light on the principles of stoichiometry, which govern the proportions in which elements combine to form compounds.
Covalent bonds, which arise from the sharing of electrons between atoms, and ionic bonds, formed through the transfer of electrons, represent the foundational mechanisms by which compounds are synthesized. The distinction between these bond types elucidates the diversity within compounds, influencing their physical characteristics, reactivity, and biological significance.
4. Molecules: The Shared Entities
Molecules are the entities formed when two or more atoms bond together, either by covalent bonds or ionic interactions. They can comprise the same type of element, such as O2 (oxygen gas), or different elements, like CO2 (carbon dioxide). Regardless of their composition, molecules remain the fundamental units in the study of chemistry, delineating the boundaries of chemical reactivity and interaction.
Furthermore, molecules can be classified based on their size and complexity. Simple molecules, comprising a few atoms, can exist as gases, whereas larger macromolecules—such as proteins and nucleic acids—are critical for biological structures and processes. The sheer diversity of molecular forms serves as a testament to nature’s ingenuity and the complex interplay of forces within chemical systems.
5. The Interrelationship Among Atoms, Molecules, Compounds, and Elements
The interconnectedness of atoms, molecules, compounds, and elements becomes apparent when examining natural phenomena. Take photosynthesis, for instance: plants convert carbon dioxide (a compound) and water into glucose and oxygen—a striking transformation facilitated by molecular interactions. These processes underscore the relational dynamics among different chemical entities—it is in their interactions that the chemistry of life unfolds.
This interplay is further accentuated by the field of quantum mechanics, which provides a deeper understanding of atomic and molecular behaviors. The probabilistic nature of electron positions and the behaviors of particles at the subatomic level enrich our comprehension of chemical interactions, suggesting that the universe is not as deterministic as classical physics might propose.
6. The Fascination with Matter
The allure of atoms, elements, compounds, and molecules lies not merely in their scientific significance but also in their profound implications for understanding the universe. The synthesis of elements in stars, the complexity of biochemical pathways, and the pursuit of new materials all stem from a fundamental curiosity about matter. Moreover, the ability to manipulate atomic and molecular structures leads to remarkable innovations in technology, medicine, and environmental science.
In conclusion, the exploration of atoms, elements, compounds, and molecules illuminates the intricate design of the universe, inviting deeper inquiries into the relationships that govern our world. Engaging with these concepts not only enhances our scientific literacy but also fosters a deeper appreciation for the complexity and beauty of the natural world.