Throughout the stark expanse of polar regions, where glacial winds howl and temperatures plunge, a remarkable phenomenon occurs: certain organisms thrive amidst the frigid embrace of ice. These entities, including fish, insects, and even some plants, harbor within their molecular architecture a fascinating class of biopolymers known as antifreeze proteins (AFPs). Their capacity to survive in subzero environments offers profound insights into not only the adaptive strategies of these organisms but also the remarkable intricacies of protein structure and function.
The resilience of life in extreme cold is a compelling observation that has piqued the curiosity of biologists and physicists alike. The antifreeze proteins that many of these organisms produce prevent the formation of ice crystals within their bodies, a phenomenon that could otherwise be lethal. To grasp how these proteins operate, one must delve deeply into the structural characteristics that define their functionality.
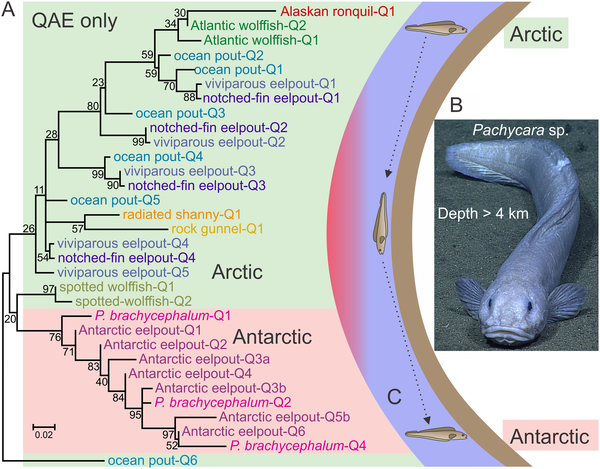
AFPs exhibit diverse structural motifs, which can be categorized into types based on their amino acid composition and three-dimensional conformation. Notably, the most well-studied AFPs include the fish type I and type II antifreeze proteins, which are characterized by a compact, globular structure. Crystallographic studies have unveiled these conformations at an atomic level, revealing intricate details about their binding interactions with ice. The structural resolution provided by X-ray crystallography facilitates a comprehensive understanding of how AFPs inhibit ice recrystallization—a critical capability that supports the survival of cold-adapted species.
The operational mechanism of antifreeze proteins is largely attributed to two interrelated phenomena: adsorption inhibition and anti-freeze activity. The molecular architecture of AFPs enables them to bind to the surface of ice crystals, effectively disrupting their growth. This interaction is governed by hydrogen bonding and hydrophobic effects, whereby specific amino acid side chains exhibit an affinity for both water and ice. As a result, the AFPs effectively provide a barrier that prevents ice from expanding within the organism’s tissues, thereby maintaining cellular integrity under extreme conditions.
In addition to their binding capabilities, antifreeze proteins display a unique property known as thermal hysteresis. This characteristic is defined as the difference in freezing and melting points of a solution. Thanks to this property, AFPs lower the freezing point of body fluids without altering the overall temperature of the organism, presenting an evolutionary advantage. The significance of thermal hysteresis is underscored by the potential applications of AFPs in industrial and biomedical sectors, where they may be employed in cryopreservation techniques, enhancing the viability of cells and tissues subjected to freezing conditions.
Furthermore, the allure of antifreeze proteins extends beyond their fundamental biochemical roles; they expose underlying principles of molecular evolution and adaptation. The ability of various species to synthesize these proteins independently illustrates an example of convergent evolution—wherein different lineages develop similar traits in response to analogous environmental pressures. This evolutionary perspective begs numerous questions about how and why such sophisticated molecular mechanisms emerged in disparate organisms responding to climate constraints.
Crystallographic investigations into the structure-function relationships of antifreeze proteins have revealed a tapestry of evolutionary innovation. For instance, the investigation of AFPs across different taxa highlights an array of structural variations, each tailored to the specific environmental niches occupied by the organisms. Some studies illustrate how certain insects possess AFPs with extended and flexible regions that enhance their efficacy in preventing ice formation during even the coldest winters, shedding light upon the adaptive significance of structural diversity.
Moreover, recent advancements in techniques such as cryo-electron microscopy further augment our understanding of antifreeze proteins, allowing for real-time observations of protein dynamics and interactions. These cutting-edge methodologies open new avenues for exploration and could unpack mechanisms that remain elusive, bestowing greater clarity upon the molecular ballet that underlies ice-proofing strategies.
In conclusion, antifreeze proteins represent a remarkable achievement of nature, enabling life to flourish beneath ice-covered surfaces and in other harsh environments. Their study through crystallography not only elucidates the physicochemical principles that underpin their antifreeze activity but also illuminates broader themes in evolutionary adaptability and molecular design. As researchers continue to uncover the secrets locked within these proteins, the implications extend far beyond mere biological curiosity; they pave the way for innovative applications across various scientific and industrial domains. Antifreeze proteins epitomize nature’s ingenuity, showcasing the delicate balance of survival amid the unforgiving harshness of polar realms.