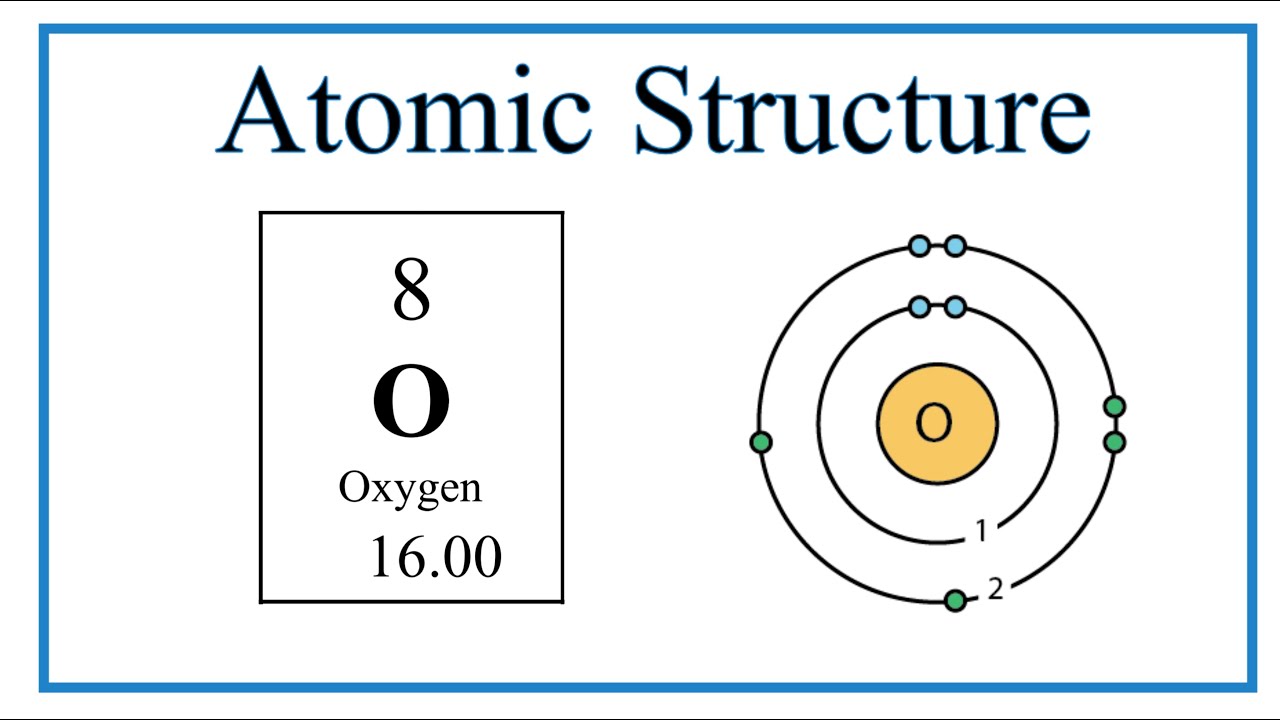
Superconductivity, a phenomenon characterized by the complete absence of electrical resistance in certain materials at low temperatures, has captivated physicists and chemists alike since its discovery in 1911. Although early research focused predominantly on metals, the exploration of various compounds—including those rich in oxygen—has revealed intriguing insights into what may be the secret ingredient behind this extraordinary state. This article delves into the essential role that oxygen plays in the superconducting properties of various materials, examining different types and the underlying mechanisms that govern their behavior.
To truly understand the relationship between oxygen and superconductivity, one must first consider the various class of superconductors, particularly the high-temperature superconductors known as cuprates. These materials, which include copper oxide compounds, have been the subject of extensive research since the discovery of high-temperature superconductivity in the 1980s. Cuprate superconductors are formed from layers of copper and oxygen, and it is hypothesized that the presence of oxygen is critical for enabling the phenomenon of superconductivity.
In the realm of cuprates, the oxygen atoms play a pivotal role in the electronic structure of these materials. Specifically, the arrangement and valence states of the oxygen atoms influence the ability of the copper atoms to participate in the formation of Cooper pairs—the pairs of electrons responsible for superconductivity. Alterations in oxygen content through doping processes or by varying the atomic arrangement not only modify the electron density but also affect the superconducting transition temperature (Tc), often improving it. Thus, oxygen emerges as a critical component in achieving high Tc in cuprate materials.
Beyond cuprates, the emergence of iron-based superconductors has further substantiated the significance of oxygen. These materials harbor layers of iron and other elements, including pnictogens and chalcogens, with oxygen commonly acting as a doping agent to enhance superconducting properties. The interplay between iron and oxygen brings forth a nuanced electron pairing mechanism distinct from that in conventional superconductors, highlighting the versatile role of oxygen in various superconducting systems.
It is pertinent to emphasize that the mechanisms underlying superconductivity are multifaceted. In many superconductors, electron-phonon interactions facilitate superconductivity, with phonons being quantized modes of lattice vibrations. In oxygen-rich materials, the vibrational modes associated with oxygen atoms can alter the electron-phonon coupling, thereby enhancing superconducting capabilities. This coupling can lead to the creation of a bosonic state that mediates attractive interactions between electrons, subsequently enabling the formation of Cooper pairs.
Moreover, the role of oxygen in solid-state superconductors extends to the structural aspects as well. The crystal symmetry and dimensionality of the superconducting material can be significantly affected by the incorporation of oxygen. For instance, the presence of oxygen can induce changes in the lattice structure, leading to either two-dimensional or three-dimensional electronic behavior. This structural diversity allows for a variety of superconducting behaviors, from conventional superconductivity in low-dimensional systems to unconventional phenomena in higher-dimensional analogs.
Examining the significance of oxygen in superconductivity inevitably leads to discussions regarding the search for room-temperature superconductors. While achieving superconductivity at ambient temperature remains an elusive goal, recent advancements in high-pressure experiments have revealed that certain hydride compounds with high oxygen content can exhibit superconductivity at temperatures above 15°C. This groundbreaking development not only emphasizes the potential of oxygen-enriched materials but also raises questions about the nature of the electron pairing mechanism in such hydrides.
While oxygen is often associated with stabilization of the electronic structure and enhancement of superconducting properties, researchers are now investigating the possibility of alternative roles that oxygen might play. For instance, studies are increasingly exploring the magnetic interactions that result from the presence of oxygen in complex crystalline structures. In some instances, it appears that oxygen vacancies can lead to modified magnetic behavior that could indirectly influence superconductivity. Understanding these multifaceted interactions is integral to future advancements in the field.
In conclusion, oxygen serves as more than just a mere additive in the context of superconductivity. Its vital contributions extend from enhancing electronic arrangements to modifying structural symmetries, all of which culminate in the emergence of superconducting states across a plethora of materials. Whether contributing to high-temperature superconductivity in cuprates, influencing iron-based superconductors, or facilitating the search for room-temperature superconductors, the role of oxygen remains a central theme in ongoing investigations. The quest to unravel the precise mechanisms by which oxygen asserts its influence continues to inspire a generation of researchers aiming to push the boundaries of superconducting materials and their potential applications.