The hydrogen atom, the simplest and most abundant element in the universe, poses intriguing questions regarding its existence, stability, and behavior when unaccompanied by other atoms. Understanding whether a hydrogen atom can exist independently invites a multifaceted examination involving quantum mechanics, atomic structure, and the fundamental principles of chemistry. This article delves into the nature of hydrogen atoms, their potential for isolated existence, and the implications of their behavior in various environments.
The Fundamental Structure of Hydrogen
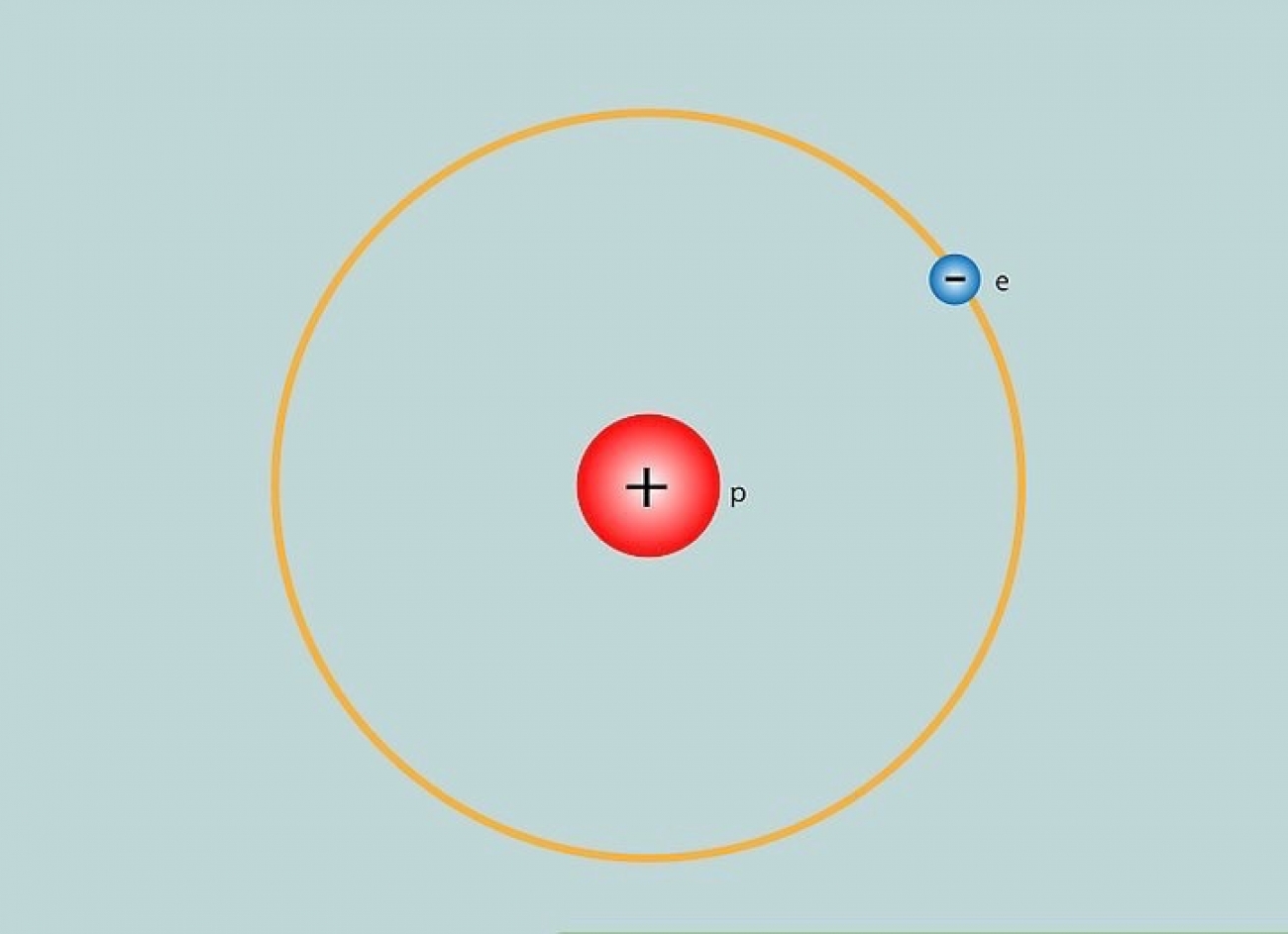
A hydrogen atom consists of one proton in its nucleus and a solitary electron in its outer shell. This simplicity makes hydrogen a popular subject of study across various scientific disciplines. The proton, with a positive charge, is balanced by the electron’s negative charge, resulting in an overall neutral atom. The atomic structure illustrates the fundamental principles of attraction and repulsion, revealing the delicate interplay of forces that govern atomic behavior.
Isolated Existence in Nature
In theory, a hydrogen atom can exist independently. In nature, hydrogen exists predominantly in molecular form (H2), where two hydrogen atoms bond together to form a diatomic molecule. This molecular configuration is favored due to the release of energy when atoms bond, resulting in greater stability. However, isolated hydrogen atoms do occur under specific conditions, notably in high-energy environments such as stars, where temperatures and pressures are sufficient to prevent molecular formation.
Quantum Mechanics and Atomic Stability
The stability of an isolated hydrogen atom is best understood through the lens of quantum mechanics. Quantum theory describes atoms as existing in probabilistic states, with electrons occupying distinct energy levels delineated by quantum numbers. An isolated hydrogen atom’s electron resides in the first energy level, the ground state, where it exhibits quantized energy behavior. Although a hydrogen atom can persist in its neutral, isolated form, the likelihood of it remaining unreacted depends on surrounding forces and energy levels.
Ionization: A Path to Uncoupled Existence
Under certain energetic conditions, hydrogen can become ionized, resulting in a proton and a free electron. Ionization occurs when sufficient energy is imparted to the hydrogen atom, such as through thermal excitation or photon absorption. In this state, the atom no longer behaves as a neutral entity but rather as a charged particle, fundamentally altering its interactions with surrounding matter. The resulting ionized hydrogen can influence chemical processes and contribute to the formation of plasma, which is ubiquitous in astrophysical phenomena.
Implications in Cosmic Environments
The cosmic landscape offers a unique context within which isolated hydrogen atoms can thrive. In the vast expanse of interstellar space, hydrogen exists predominantly as individual atoms. This atomic hydrogen plays a significant role in astrophysics, serving as a crucial component in the formation of stars and galaxies. The conditions within star-forming regions often allow hydrogen to exist in isolated states, where it cools and condenses under gravitational attraction, eventually leading to nuclear fusion within stellar cores.
The Role of Temperature and Pressure
Temperature and pressure are critical determinants of hydrogen’s capacity to exist independently. At extremely high temperatures (millions of degrees Celsius), such as those found in the core of stars, hydrogen atoms are frequently found in isolated states, contributing to nuclear fusion processes that power stars. Conversely, under standard atmospheric conditions on Earth, hydrogen primarily exists as diatomic molecules due to intermolecular attractions that favor molecular formation.
Experimental Perspectives
Experimental physicists have developed methodologies to isolate hydrogen atoms in controlled laboratory settings. Techniques such as laser cooling and magnetic confinement allow researchers to study the properties of single hydrogen atoms. These investigations reveal insights into atomic behavior, such as electron spin and quantum state transitions, yielding valuable knowledge that enhances our understanding of atomic physics as a whole.
Dynamic Interactions and Chemical Reactions
The reactivity of hydrogen plays a pivotal role in its existence within various chemical processes. While an isolated hydrogen atom can maintain its individual status, it is often reintroduced into molecular frameworks through chemical reactions. Hydrogen readily participates in reactions with more electronegative elements, forming stable compounds such as water (H2O) and hydrocarbons. These interactions underscore the atom’s versatility and the ease with which it transitions from a free particle to a molecular constituent.
Conclusion: A Dual Existence
In conclusion, it is indeed possible for hydrogen atoms to exist independently under certain conditions, primarily within environments characterized by high energy, low density, or significant gravitational forces. Nonetheless, the predominance of hydrogen in diatomic form in naturally occurring settings underscores the intricate balance between atomic stability and molecular formation. The capacity for hydrogen to exist as a singular atom provides profound implications for various scientific fields, from cosmology to chemistry, highlighting its significance as a fundamental building block of matter in the universe.