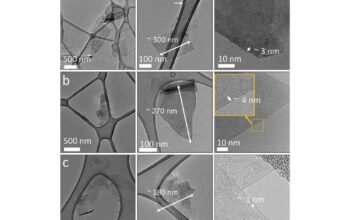
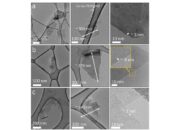
Boron nitride (BN) is a fascinating compound characterized by its unique properties and diverse applications. Often likened to graphite in its structure but with completely different attributes, boron nitride is a covalent network solid that exhibits remarkable thermal and chemical stability. To appreciate the intriguing interactions that occur within this compound, it is essential to delve into the electron-sharing mechanisms between its constituent elements: boron and nitrogen.
Understanding the Atomic Structure of Boron and Nitrogen
Boron, with an atomic number of 5, possesses three valence electrons in its outer shell. This incomplete octet compels boron to seek additional electrons to achieve a stable electronic configuration. Nitrogen, on the other hand, with an atomic number of 7, has five valence electrons. The quest for stability drives nitrogen to gain three additional electrons to fulfill its octet rule. This inherent disparity in electron configuration prompts an examination of how boron and nitrogen engage in the sharing of electrons within boron nitride.
Electron Sharing: Bridging the Gap
The formation of boron nitride entails a covalent bond due to the mutual electron sharing between boron and nitrogen. This process is particularly intriguing because it raises a question: How do these two elements, with their differing electronegativities—boron being less electronegative than nitrogen—achieve a stable compound? The answer lies in the formation of a three-dimensional network where boron and nitrogen atoms interlace through shared pairs of electrons.
When boron and nitrogen combine, they form a structure reminiscent of graphite, where layers of atoms are bound together by strong covalent bonds. Each boron atom shares electrons with nitrogen atoms to form BN units across the lattice. In this configuration, the boron atom contributes its three valence electrons, while nitrogen contributes its three. Consequently, each boron atom forms bonds with three nitrogen atoms, creating a hexagonal array typical of the layered structure of BN.
The Role of Hybridization in Boron Nitride
Examining the hybridization concept is pivotal in understanding the bonding behavior in boron nitride. Boron typically undergoes sp2 hybridization, allowing it to form three sigma bonds in a planar arrangement. This hybridization facilitates the creation of a stable bond with nitrogen atoms, which also align to maintain a planar configuration. On the other hand, nitrogen atoms engage in the formation of p orbitals, contributing to the overall efficacy of the bonding paradigm.
This spatial arrangement leads to unique structural characteristics. The alternating layers of boron and nitrogen give rise to the compound’s impressive mechanical and thermal properties. In essence, the electron-sharing phenomenon not only establishes chemical bonds but also underpins the resultant material’s functionality.
Polar and Nonpolar Characteristics of Boron Nitride
One might wonder about the resulting polarity of the bonds formed between boron and nitrogen. Given that nitrogen is more electronegative than boron, this disparity results in a polar covalent bond. However, due to the symmetrical arrangement of the BN lattice, the overall structure exhibits a nonpolar character. This subtle juxtaposition of polar bond nature versus overall symmetry produces intriguing outcomes regarding the material’s behavior in various environments, particularly as an insulator in electronic applications.
Applications Driven by Electron Sharing Phenomenon
The implications of the boron-nitrogen bond extend far beyond simple electron sharing; they manifest in multiple applications essential to contemporary technology. Boron nitride’s impressive thermal stability and excellent electrical insulating properties make it an optimal choice for components in semiconductor devices. Furthermore, its lubricating qualities are harnessed in numerous industrial applications, alleviating friction and wear.
Moreover, the uniquely structured boron nitride plays a significant role in cosmetics, where its inertness and smoothness enhance the texture and application of various products. A daunting challenge lies in harnessing these properties fully, as researchers continuously explore novel synthesis techniques and structures that can provide even more enhanced functionalities.
The Future of Boron Nitride Research
As research progresses, the potential for novel uses of boron nitride appears limitless. The ability to manipulate and optimize the electron-sharing mechanisms within BN may lead to advancements in nanotechnology, photonic devices, and even biomedical applications. This emerging paradigm shift poses critical questions regarding the future of materials science and challenges researchers to unlock the compound’s full potential.
In conclusion, the interaction between boron and nitrogen in boron nitride is a subject that intertwines fundamental chemistry with cutting-edge applications. The nuanced interplay of electron sharing leads to the creation of a material that not only defies expectations but also serves as a testament to the beauty of scientific endeavor. As this field continues to evolve, one can only speculate about the revolutionary applications that may emerge from a deeper understanding of its underlying principles.