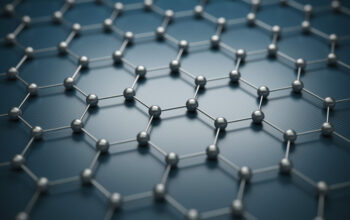
Graphene, a remarkable material composed of a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, has captivated the attention of scientists, engineers, and manufacturers alike since its isolation in 2004. Born from the elemental simplicity of carbon, the substance is imbued with extraordinary properties, leading to widespread inquiries regarding its synthesis and potential applications. This leads us to the poignant question: can you make graphene from carbon atoms?
The allure of graphene stems from its unparalleled attributes. It possesses exceptional electrical conductivity, mechanical strength—estimated to be over 100 times stronger than steel—and remarkable thermal conductivity. These properties have galvanized research into a myriad of potential applications, ranging from flexible electronics to biomedical devices. Yet, the journey from simple carbon atoms to this complex structure is anything but straightforward.
To understand the feasibility of creating graphene from carbon, one must first appreciate the nature of carbon itself. Carbon is a versatile element, capable of forming a variety of allotropes, each exhibiting distinct physical properties. Among these allotropes are graphite, diamond, and fullerenes. Each configuration results from the specific arrangement of carbon atoms and the types of bonds formed between them.
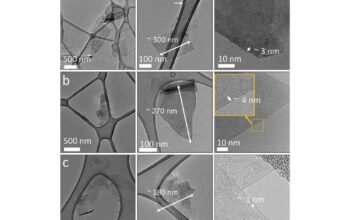
In essence, graphene can indeed be synthesized from carbon atoms, but the manner of this synthesis is crucial. Various methods exist, each involving different mechanisms and pathways. The most common techniques employed in the production of graphene include mechanical exfoliation, chemical vapor deposition (CVD), liquid-phase exfoliation, and chemical reduction of graphene oxide, among others.
Mechanical exfoliation, often referred to as the “Scotch tape method,” is perhaps the most illustrative technique. This process involves peeling away layers of graphite to obtain single or few-layer graphene sheets. The simplicity of this method belies the inherent challenges, as achieving uniformity and scalability remains problematic. While this technique effectively yields high-quality graphene, its application is limited to laboratory-scale production.
Alternatively, chemical vapor deposition (CVD) offers a more scalable approach. In CVD, a gaseous carbon source, such as methane, is decomposed on a substrate at elevated temperatures, allowing carbon atoms to precipitate and self-assemble into a graphene layer. This method not only facilitates the growth of larger graphene sheets but also enables the integration of graphene with various substrates—critical for numerous electronic applications. Nevertheless, CVD is often accompanied by complexities in substrate selection and process optimization.
Another promising method for generating graphene is the liquid-phase exfoliation of graphite, wherein graphite is subjected to ultrasonication in a solvent, leading to the dispersion of graphene in the liquid medium. This approach can produce large quantities of graphene, albeit often in smaller, less uniform flakes, which may limit its applicability for certain high-performance uses. However, the flexibility of processing attributed to liquid-phase methods opens avenues in diverse industries, such as composite materials and coatings.
The chemical reduction of graphene oxide presents another intriguing pathway. Graphene oxide, derived from the oxidation of graphite, is more amenable to dispersion in solvents due to the presence of functional groups. Through reduction processes, chemically or thermally, graphene oxide can be transformed into reduced graphene oxide, showcasing properties more akin to its pristine form. However, this process may introduce defects or residual functional groups, affecting the overall properties of the resulting material.
While the aforementioned methods demonstrate the capacity to convert carbon into graphene, the intrinsic qualities of the synthesized material may vary significantly based on the chosen production technique. These variations pose critical considerations for researchers and engineers aiming to exploit graphene’s attributes for commercial applications. Impurities, defects, and structural integrity are paramount; they can dictate performance outcomes in electronic, photonic, and mechanical systems.
Graphene’s potential extends beyond mere industrial applications; it sparks profound philosophical inquiries into materials science and nanotechnology. The transition from simple carbon atoms to a complex two-dimensional material encapsulates a broader narrative of transformation and innovation. As scientists continue to unlock the mysteries surrounding graphene, they contemplate the implications of such a versatile material on sustainable technology, energy storage, and the future of computing.
Moreover, the fascination surrounding graphene is accentuated by its alignment with contemporary trends toward miniaturization and efficiency. In an era where technological advances require materials that can deliver performance without compromising sustainability, graphene presents a tantalizing solution. Its potential to enhance battery life, increase the efficiency of solar cells, and create ultralight structures underscores its role in fostering a new generation of advanced materials.
In conclusion, while it is indeed possible to create graphene from carbon atoms through various synthesis techniques, the nuances of each methodology significantly influence the material properties and practical applications. The journey from raw carbon to graphene is not merely a transformation of element to material but rather a reflection of human ingenuity and the seamless interplay of fundamental science and engineering prowess. As research progresses, the continued exploration of graphene synthesis will undoubtedly unveil new dimensions of this remarkable material, propelling it into realms previously unimagined.