Graphene, often heralded as a “wonder material,” represents an extraordinary slice of the material universe, akin to a pristine diamond concealed within a rock. Its remarkable properties—astonishing strength, unparalleled electrical conductivity, and immense surface area—render it not merely a novel substance but a revolutionary platform for technological advancements. Given these alluring characteristics, a question lingers: can anyone make graphene in a tube?
To grapple with this inquiry, one must first recognize what graphene embodies. Graphene consists of a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice. Beyond its structural simplicity lies a depth of complexity, where each atom bridges the realms of chemistry and physics, offering fertile ground for innovation. Yet, while the synthesis of graphene may seem tantalizingly within reach of any curious enthusiast, the reality is considerably more nuanced.
Let us embark on an exploration of the zealous pursuit of graphene, starting with a few common methodologies employed in its synthesis. One prominent technique is the mechanical exfoliation method, also dubbed as the “Scotch tape method.” This technique metaphorically likens the creation of graphene to peeling layers off an onion. Through this delicate process, layers of graphite can be precisely isolated until singular layers of graphene are liberated. While this method is remarkably instructive and can yield high-quality graphene, it is not without limitations in scalability and uniformity, posing challenges for widespread application.
Another prominent synthesis technique is chemical vapor deposition (CVD), akin to painting on a canvas. Here, carbon-containing gases are introduced to a substrate, leading to the deposition of graphene. This method not only facilitates the production of larger quantities but also enhances the quality of the produced graphene. Yet, the setup requires sophisticated equipment and a delicate understanding of reaction parameters—conditions like temperature and gas flow must be meticulously controlled to avoid impurities. For the amateur or the uninitiated, the curtain of complexity can be daunting.
A more accessible alternative might be liquid-phase exfoliation, reminiscent of a culinary process where ingredients are blended together to extract flavors. By dispersing graphite in a solvent and applying ultrasonic waves, layers can be separated into nanosheets of graphene. While simplified in concept, achieving high yields and maintaining the integrity of the graphene involves navigating a labyrinth of variables—factors that can often baffle the novice experimenter.
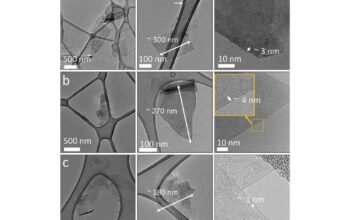
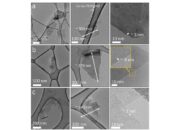
The allure of **tubular graphene structures**, or graphene oxide tubes, presents yet another layer of intrigue. These nanostructures possess unique properties and open avenues for applications ranging from drug delivery to nanocomposites. The synthesis of such structures typically demands higher control over chemical reactions and extensive knowledge of material science. For the untrained eye, producing tubular graphene seems nearly as speculative as trying to sculpt the Sistine Chapel’s ceiling with one’s bare hands.
What emerges from this synthesis exploration is the stark reality: although the dream of making graphene in a tube tantalizes many, the pursuit is shrouded in complexities that are not readily graspable by all. The endeavor requires not just materials and equipment, but also an intimate understanding of the underlying science. To conjure graphene from the ether of scientific imagination into tangible reality necessitates a blend of passion, precision, and expertise.
However, the accessibility of knowledge and resources in today’s digital age suggests a promising horizon. Online platforms, academia, and maker communities are revered bastions wherein aspiring experimenters can challenge the boundaries of their craft. Tutorials abound, offering guidance through the labyrinth of graphene synthesis. Additionally, a plethora of DIY kits is now available, aimed at both educational institutions and individual enthusiasts. Such resources serve as beacons, illuminating the path toward graphene production. In this sense, the question becomes less about the ability to make graphene and more about one’s commitment to learning and experimenting.
Moreover, the implications of successful graphene synthesis extend far beyond the confines of scientific curiosity. As industries—from electronics to renewable energy—stand on the precipice of transformation, the demand for graphene rises like an insatiable tide. Practical applications burgeon, with potential implications in fields as diverse as medicine, where graphene’s biocompatibility could lead to groundbreaking therapeutic interventions, to aerospace engineering, where its lightweight toughness could revolutionize material selection.
As our understanding of graphene evolves, so too does the potential for crowdsourced innovation. The fusion of disparate ideas conjures a fertile ground for collaborative projects, where the collective wisdom of the masses could yield previously unimaginable breakthroughs. The metaphorical tube through which graphene is synthesized may serve as a conduit not just for individual learning, but also for advancing a communal quest for knowledge. In this context, even those who lack formal training might find themselves contributing to the growing tapestry of graphene research and production.
In conclusion, the question “Can anyone make graphene in a tube?” invites a wealth of consideration. While the practical hurdles of producing graphene are nontrivial—like navigating a maze blindfolded—the potential for democratizing this wondrous material remains tantalizingly within grasp. With passion, perseverance, and nurturing collaboration, those who dare to venture into the world of graphene synthesis may well uncover its mysteries, transforming aspirations into reality within the metaphorical tubes of creation.