Imagine a world where everyday materials become extraordinary, where the mundane transforms into the miraculous. What if a simple impurity could elevate a solid from a mere insulator to a luminous conductor of electricity? This phenomenon—known as doping—poses a tantalizing challenge in the realm of solid-state physics and materials science. The process of introducing impurities into a material fundamentally alters its electronic properties, enabling the creation of semiconductors that form the backbone of modern technology. Let us delve into the intricacies of doping and explore how it transforms ordinary solids into the superstars of the materials world.
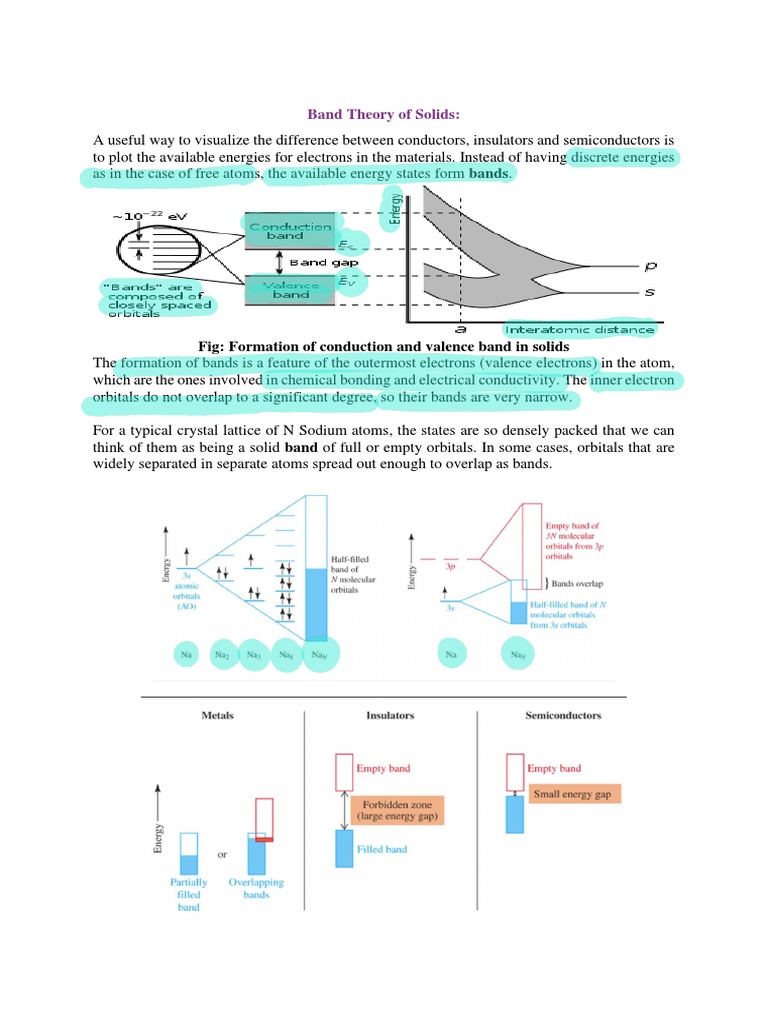
To commence, we must understand the basic structure of solids. At the atomic level, solids consist of an arrangement of atoms, forming a lattice structure that defines their unique properties. The electrons within these atoms occupy specific energy levels, which are critical in determining whether a material acts as a conductor, insulator, or semiconductor. In pristine, undoped semiconductors, the electrons exist in a valence band, while the conduction band, which enables electrical conduction, lies above it—in an ‘inactive’ state. The gap between these bands, known as the band gap, represents the energy required to excite electrons into the conduction band.
Now, what happens when we introduce impurities into this electronic ecosystem? Doping modifies the band structure by adding or subtracting electrons from the material, effectively manipulating the band gap. This injection of foreign atoms can create additional energy levels within the band gap or alter the distribution of the existing energy bands, resulting in significant changes to conductivity. This capability is harnessed in various applications ranging from transistors to solar cells.
Two principal types of doping exist: n-type and p-type. N-type doping involves adding donor atoms—commonly elements such as phosphorus into silicon. These donor atoms possess more valence electrons than the host material; hence, they provide extra electrons that occupy energy levels just below the conduction band. This results in an increase in electron concentration, which enhances the material’s ability to conduct electricity. In contrast, p-type doping introduces acceptor atoms, such as boron, which have fewer valence electrons than the semiconductor. These acceptor atoms create ‘holes’ in the valence band, allowing electrons to move and effectively increasing the material’s hole concentration. The movement of these holes contributes to electrical conduction, albeit through a different mechanism than n-type materials.
Consider the remarkable transformation seen in the doping process. A pure silicon crystal displays modest conductivity at room temperature, but with strategic doping, its electrical characteristics can be drastically enhanced. This phenomenon underpins the operation of billions of devices globally, including diodes and transistors, which are the fundamental building blocks of digital technology. However, such transformations are not without their intricacies. The concentration of dopants, the temperature at which doping occurs, and the crystallographic orientation of the host material can all significantly affect the outcome. The precision of doping techniques, such as ion implantation and diffusion, can dictate the success of the desired traits in the resulting materials.
As we delve deeper into the implications of doping, we encounter another layer—one that reflects the dynamism of material properties. The interplay between doping and temperature illustrates an enthralling relationship. Although the conductivity of doped semiconductors generally increases with temperature, there are thresholds where increased thermal energy can cause scattering of charge carriers, thus affecting performance. This duality creates a fascinating challenge in tailoring materials for specific applications. Engineers and scientists must carefully balance the level of doping and operational conditions to achieve optimal performance in real-world environments.
Moreover, the role of doping extends into the burgeoning field of advanced materials—graphene and transition metal dichalcogenides (TMDs), for example. These materials exhibit extraordinary electronic, optical, and mechanical properties, making them candidates for the next generation of electronics. Doping in such materials can lead to modifications in their electrical and optical characteristics, opening new avenues for innovation. The challenge thus becomes one of navigating these novel landscapes—understanding how traditional doping principles apply, or don’t, to these two-dimensional materials.
But can we push the boundaries even further? The pursuit of higher doping levels poses potential complications that researchers are beginning to explore. Too many impurities can result in scattering effects and material degradation, ultimately diminishing conductivity gains. Here lies an invitation for ingenuity: How can scientists develop new methods for doping that enhance conductivity without compromising the structural integrity of the material?
Emerging techniques such as self-assembly and molecular beam epitaxy show promise in achieving this delicate balance. By enabling precise control over dopant introduction and distribution, these innovative methods could facilitate the creation of superior materials with tailored properties. The aspiration is not just to create effective semiconductors but to revolutionize how we think about and utilize materials in technology.
In conclusion, doping serves as an essential mechanism when transforming solids into superstars of conductivity. The alteration of electronic properties through the infusion of impurities holds significant implications across various fields, demonstrating the intersection of chemistry and physics. As we continue to explore the challenges and complexities associated with doping, the quest for optimizing material performance remains at the forefront of scientific inquiry. The journey from mundane to exceptional lies in our ability to manipulate the invisible forces at play, paving the way for future advancements in technology and materials science.