In the realm of advanced materials, borophene emerges as a remarkable contender that transcends previous benchmarks in both strength and flexibility, akin to a phoenix rising from the ashes of traditional two-dimensional materials such as graphene. This cutting-edge monolayer of boron, characterized by its intricate atomic lattice, garners significant attention due to its exceptional mechanical properties and potential applications in various technology sectors. Here, we delve into the captivating world of borophene, exploring its unique structural attributes, mechanical resilience, and the implications of its unprecedented strength at the atomic scale.
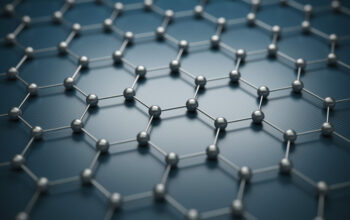
To appreciate how strong borophene truly is, one must first delve into its atomic structure. Borophene is composed of boron atoms arranged in a two-dimensional honeycomb lattice, wherein each boron atom connects via covalent bonds. This arrangement not only imparts distinctive properties to borophene, but also sets it apart from its boron allotropes and other two-dimensional materials. The coordination of boron atoms within the lattice creates a range of bonding configurations that yield a composite structure, empowering borophene with unprecedented stability and mechanical strength.
One of the defining characteristics of borophene is its remarkable tensile strength, which exceeds that of graphene, a material long heralded for its robustness. The tensile strength of borophene has been approximated to reach values of 130 GPa, while graphene stands at about 100 GPa. This potential for borophene to withstand higher applied forces without undergoing failure establishes it as a benchmark material for future applications in electronics, nanotechnology, and structural engineering. The impressive strength-to-weight ratio of borophene is reminiscent of a spider’s silk, which exhibits extraordinary tensile properties despite its lightweight nature, captivating researchers and industry professionals alike.
Flexibility is another hallmark trait of borophene. Unlike conventional materials that exhibit significant rigidity, borophene can twist and bend, allowing for versatile applications in flexible electronics and nanoscale devices. When subjected to external strain, borophene maintains its mechanical integrity, showcasing an ability to recover its original shape after deformation. This adaptability parallels a gymnast’s ability to execute a flawless routine amidst a dynamic environment, further emphasizing the material’s unique potential in practical applications.
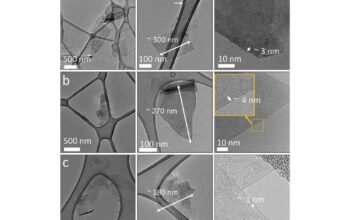
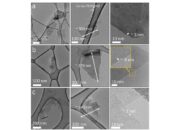
The synthesis of borophene has also spurred interest in its mechanical attributes. Researchers have developed various methodologies for fabricating borophene monolayers, including chemical vapor deposition and liquid-phase exfoliation techniques. These innovative processes not only yield high-quality borophene but also enable the exploration of its superior mechanical properties on a macroscopic scale. The advancement of synthesis technologies further illustrates the transformative potential of borophene and its viability for industrial applications.
Moreover, borophene’s inherent properties extend beyond mere strength and flexibility; they encompass remarkable thermal and electrical conductivity. The complex interplay between conductivity and structural features within borophene enables it to conduct electricity with exceptional efficiency. Its unique electronic structure is defined by a wealth of available states near the Fermi level, setting the stage for remarkable performance in electronic components. Potential applications include high-performance transistors, fine-tuned sensors, and energy storage systems that outperform existing materials on the market.
As the exploration of borophene continues, one cannot overlook the burgeoning interest in its oxidation process. The ability to manipulate and control borophene’s oxidation state holds the key to unlocking even more advanced properties. In a world where environmental considerations gain increasing importance, understanding the oxidation of borophene could pave the way for sustainable material development. The adaptive characteristics exhibited by borophene once oxidized suggest potential utilization in catalysis and energy conversion applications—transforming a child of the universe into a tool for ecological advancement.
Furthermore, one must also consider the implications of borophene’s unparalleled strength at the atomic scale. It serves as a testimony to the ongoing revolution within the materials science landscape, where materials previously thought to possess insurmountable limitations are being redefined. The exploration of borophene embodies a paradigm shift, blending the realms of theoretical and practical applications. As researchers uncover new pathways to harness its properties, the scope of what is deemed possible extends exponentially.
In conclusion, borophene stands as an emblem of ingenuity at the atomic scale. Its strong, flexible, and conductive attributes beckon a re-evaluation of material capabilities within the modern technological landscape. The synthesis, properties, and potential applications of borophene invite intrigue, anticipation, and excitement for what lies ahead. In the pursuit of innovative solutions to contemporary challenges, borophene emerges not only as a marvel of material science but as a beacon of future technological advancements. As investigations into its mechanics and applications unfold, we unveil the profound potential harbored within this atomic wonder; a new era of materials is on the horizon, ushered in by the formidable strength of borophene.