In the realm of physics and materials science, the quest for understanding the properties of matter has led to remarkable discoveries in the microscopic world. One of the most captivating questions in this domain is: how thick is an atom? At first glance, this query appears deceptively simple; however, it opens a gateway to the intriguing characteristics of two-dimensional (2D) crystals and the implications therein.
To embark upon this exploration, it is imperative to delineate the concept of thickness in the atomic world. An atom itself is not a solid entity with quantifiable dimensions akin to macroscopic objects. Rather, it comprises a nucleus of protons and neutrons surrounded by a cloud of electrons, which exhibit wave-particle duality. Thus, when we refer to the “size” of an atom, we often speak in terms of atomic radius, a measurement that is often influenced by the environment in which the atom exists.
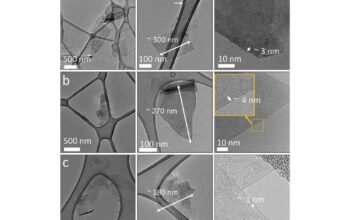
The notion of 2D crystals presents a paradoxical concept that further complicates the question of atomic thickness. These materials exhibit astonishing properties and are composed of a single or few layers of atoms arranged in a crystalline lattice. Graphene, for example, is the archetype of 2D materials, consisting of a single layer of carbon atoms arranged in a hexagonal pattern. The thickness of graphene is scarcely the thickness of a single atomic layer, approximately 0.34 nanometers. This minuscule measurement embodies the essence of nanotechnology and illustrates the thin boundaries that define our understanding of matter.
The discovery of graphene, however, has spurred a renaissance in the exploration of other 2D materials. Transition metal dichalcogenides (TMDs), such as molybdenum disulfide (MoS2), have drawn significant attention due to their remarkable electronic and optical properties, which differ markedly from their bulk counterparts. For instance, while bulk MoS2 is an indirect bandgap semiconductor, monolayer MoS2 transitions to a direct bandgap semiconductor, thereby enhancing its applicability in optoelectronic devices.
As we delve deeper into the physical characteristics of these materials, we encounter the fascinating concept of quantum confinement. In lower-dimensional systems, confined electrons exhibit distinct energy states, which alters their conductive properties. This phenomenon is particularly pronounced in 2D crystals, where the electronic behavior can be manipulated by altering the layer’s thickness. The thickness of an atom—albeit abstract—becomes pivotal in determining the functional capabilities of these novel materials.
The inquiry does not merely end with the thickness of individual atoms, as the stacking of these 2D crystals into heterostructures introduces yet another layer of complexity. These heterostructures, composed of different 2D materials, can exhibit emergent properties that are not present in the individual components. Such exceptional properties typically arise from interlayer interactions, which can be finely tuned through various methodologies, including mechanical exfoliation or chemical vapor deposition. Consequently, the concept of thickness expands to include layer interactions and material interfaces, emphasizing the intricate tapestry of atomic organization.
Further compounding this dialogue is the implication of Van der Waals forces, which govern the interaction amongst layers in these stacked systems. The weak nature of these forces enables the strata to slide over one another with relative ease, a feature that underlies the exceptional flexibility and strength of materials like graphene. This remarkable property manifests in various potential applications, including flexible electronics and high-strength composites.
Moreover, the synthesis of 2D materials and the engineering of their thickness could herald advancements in the burgeoning field of two-dimensional electronics. The reduced dimensionality not only facilitates miniaturization but also opens the door to novel electronic architectures. The ability to tailor electrical, thermal, and optical properties at the atomic level could revolutionize technologies, giving rise to ultra-efficient devices and energy solutions.
One of the most alluring aspects of the investigation into 2D crystals is the potential for these materials to enhance energy storage and conversion mechanisms. The exploration of atomic thickness leads to innovations in batteries and supercapacitors. By leveraging the high surface area and tunable properties of 2D materials, researchers are forging pathways for energy solutions that could reshape our approach to sustainability.
In essence, the question “How thick is an atom?” transcends the confines of a mere scientific inquiry; it serves as a lens through which the grandeur of modern materials science can be appreciated. The evolution of thought surrounding atomic structure and the concept of thickness invites a paradigm shift in our understanding of matter at the nanoscale. Each discovery sheds light on the realities that govern our material world and compels some to explore synthetic methodologies, applications, and potentials previously inaccessible.
In conclusion, the intriguing case of 2D crystals illustrates not only the complexity that arises when examining atomic thickness but also the profound implications that such knowledge entails. As research advances and our comprehension deepens, the once esoteric notions surrounding the dimensions of atoms move closer to tangible applications that promise to revolutionize technology and reshape everyday life. The convergence of atomic science with practical utility ignites curiosity and emboldens the scientific community to further unveil the enigmatic universe of materials that lie just beyond our perception.