The scientific intrigue surrounding graphene and graphite rests fundamentally on their structural composition and the implications of these structures on their respective properties and applications. To the untrained eye, these two carbon allotropes may seem interchangeable, yet, they represent a profound divergence in both characteristics and potential uses. This exploration dissects their differences, not merely to inform but to invigorate curiosity about the versatile realm of carbon materials.
1. Structural Composition: Atoms and Layers
Graphite is characterized by a three-dimensional arrangement of carbon atoms. Each carbon atom within graphite is covalently bonded to three adjacent atoms in a planar structure, forming hexagonal sheets. These sheets are then interleaved with weak van der Waals forces, allowing for facile slip between layers. Thus, graphite exhibits anisotropic properties— a quality that enables it to conduct electricity in the plane of the sheets while remaining an electrical insulator out of plane.
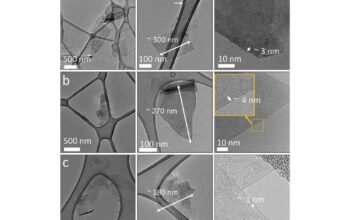
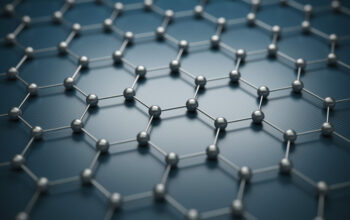
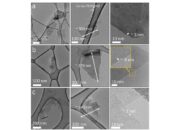
Conversely, graphene is essentially a single monolayer of carbon atoms arranged in a two-dimensional lattice. Graphene’s structure can be visualized as a honeycomb configuration, granting it unparalleled strength, with experimental evidence suggesting it can be up to 200 times stronger than steel. This unique arrangement catalyzes a multitude of remarkable physical properties, some of which have piqued scientific interest.
2. Physical Characteristics: Strength, Flexibility, and Conductivity
The disparity in structural forms translates to significant differences in the physical characteristics of graphene and graphite. Graphene possesses exceptional mechanical properties. Its tensile strength, coupled with impressive flexibility, makes it an attractive candidate for a multitude of applications, from flexible electronics to advanced composite materials.
Graphite, whilst structurally robust, lacks the single-layer elegance of graphene. It is primarily used in applications requiring high thermal and electrical conductivity. In bulk form, graphite is a lubricant and is widely utilized in batteries and nuclear reactors. However, it does not exhibit the same degree of electrical conductivity per unit area when compared to graphene, primarily due to the dimensional constraints imposed by its layered structure.
3. Electrical Conductivity: A Superconductor vs. an Electrode
Electrical conductivity emerges as a pivotal point of differentiation between these two carbon allotropes. Graphene showcases an extraordinary level of electrical conductivity, with an intrinsic carrier mobility that far surpasses traditional conductors. This capability has prompted intense research into its potential applications in next-generation electronics, such as supercapacitors and transistors that operate at exceptional speeds.
Graphite, although conductive—particularly along its planes—is limited compared to graphene. Its layered nature introduces a level of charge carrier scattering which diminishes overall conductivity. Thus, while graphite can function effectively as an electrode material, graphene’s unparalleled conductivity opens avenues for advancements in high-performance electronic devices.
4. Thermal Properties: Conductivity and Management
Thermal management is another arena where graphene takes precedence. Featuring remarkably high thermal conductivity, graphene allows for efficient heat dissipation, making it a prime candidate for applications in thermally sensitive electronics. The unique two-dimensional structure facilitates rapid energy transfer, minimizing hot spots and enhancing device longevity.
On the other hand, graphite’s thermal conductivity is dictated by its layered structure. While it performs admirably as a thermal conductor, it remains less efficient than graphene, particularly in applications requiring rapid heat dissipation. Understanding these thermal disparities is essential when selecting materials tailored to specific industrial needs.
5. Optical Properties: Transparency and Efficiency
The optical characteristics of graphene further illuminate its distinction from graphite. Graphene is nearly transparent, absorbing only about 2.3% of visible light across a broad range of wavelengths. This feature renders it a compelling candidate for use in transparent conductive films and other optoelectronic devices, fostering innovation in display technologies.
In stark contrast, graphite is opaque due to its multilayered structure which scatters light. This opacity limits its use in optical applications, relegating it primarily to uses in bulk materials where light interaction is minimal. The ability of graphene to combine transparency with conductivity positions it at the forefront of both scientific research and commercial application.
6. Chemical Reactivity and Functionalization
The chemical reactivity of graphene contrasts robustly with that of graphite, laying the groundwork for further functionalization. Graphene’s surface area, coupled with its reactive nature, allows for numerous opportunities to modify its properties through chemical functionalization. Researchers explore these possibilities extensively, aiming to tailor graphene for enhanced performance in applications ranging from drug delivery systems to advanced sensors.
Graphite, while chemically stable, lacks the same level of reactivity as graphene. Its bulk form often limits the scope of chemical interactions, hindering possibilities for functional enhancement. Understanding how chemical alterations can influence performance outcomes is critical for materials scientists and engineers alike.
7. Future Implications: A Paradigm Shift
As we stand on the precipice of significant breakthroughs in materials science, the implications of understanding the differences between graphene and graphite resonate throughout various fields. The unique properties of graphene promise to revolutionize sectors including nanotechnology, electronics, and materials engineering, allowing for the creation of innovative devices that exhibit extraordinary performance characteristics.
In conclusion, while both graphene and graphite stem from the same elemental foundation, their distinct structural and physical attributes yield a wealth of possibilities. The extraordinary properties of graphene allure both researchers and industry professionals, promising not merely advances in technology but a veritable shift in perspective on the potential of carbon-based materials. As curiosity leads to exploration, the future of materials science may well hinge on these remarkable carbon allotropes, and the stories they continue to unfold are only just beginning.