Graphene, a two-dimensional allotrope of carbon, has garnered a tremendous amount of attention in both academic and industrial domains due to its remarkable properties. This article explores the nature of graphene’s composition, specifically addressing whether graphene is synthesized from carbon sources or directly from carbon atoms. To gauge this relationship, it is essential to understand the underlying principles of graphene formation, the different methods employed in its synthesis, and the implications of its atomic structure.
Understanding Graphene’s Structure
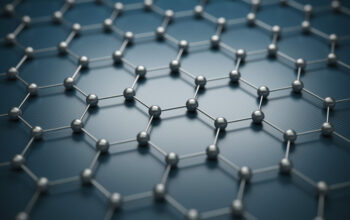
Graphene is principally composed of carbon atoms arranged in a hexagonal lattice. The unique arrangement allows for exceptional mechanical strength, electrical conductivity, and thermal properties. Each carbon atom is sp2 hybridized, forming three sigma bonds with adjacent carbon atoms while participating in a delocalized π-bond system. This configuration is pivotal in underpinning graphene’s unique qualities, facilitating the free movement of electrons across its surface and contributing to its remarkable durability.
Synthetic Pathways for Graphene Production
Graphene can be synthesized through various methodologies, each leveraging the fundamental properties of carbon. The three predominant approaches include mechanical exfoliation, chemical vapor deposition (CVD), and liquid-phase exfoliation.
1. Mechanical Exfoliation
The mechanical exfoliation method, often hailed for its simplicity, involves peeling layers of graphite to obtain thin sheets of graphene. This technique capitalizes on the weak van der Waals forces between graphite layers, resulting in a material that remains largely in its atomic structure while achieving the desired two-dimensional morphology.
2. Chemical Vapor Deposition (CVD)
CVD is a more sophisticated approach that involves heating gaseous precursors containing carbon—such as methane—at elevated temperatures. The carbon atoms are deposited on a substrate, where they rearrange themselves to form a continuous graphene layer. This method allows for high-quality graphene production, enabling tailored properties through the choice of substrates and growth conditions.
3. Liquid-Phase Exfoliation
This technique employs sonication and shear forces to disperse graphite into a solvent. The graphite flakes are broken down into nanosheets, yielding smaller graphene structures. While this method may produce lower-quality graphene, it is advantageous for large-scale production and integration into composite materials.
The Role of Carbon Sources
Throughout these synthetic methods, the source of carbon plays a critical role. Graphene is invariably derived from carbon atoms. Thus, the query “Do you make graphene out of carbon or from carbon atoms?” can be succinctly answered: graphene is fundamentally constructed from carbon atoms, which are manipulated through various technological processes to yield graphene sheets.
Implications of Graphene’s Atomic Structure
Understanding graphene’s atomic composition has profound implications across multiple domains. The ability to manipulate carbon at the atomic level opens avenues for applications ranging from electronics to biomedicine. In electronics, graphene’s exceptional conductivity and strength suggest potential for flexible and high-performance electronic devices. Moreover, in biomedicine, graphene’s biocompatibility and surface properties allow for drug delivery systems and biosensors. Its application in composite materials enhances tensile strength while reducing weight, making it an attractive candidate for use in aerospace and automotive industries.
Environmental Considerations
As interest in graphene expands, so does concern about the environmental implications of its production. Several synthesized processes, particularly those involving chemical vapor deposition, may utilize toxic precursors or generate hazardous waste. Researchers are keen to develop greener synthesis methods that minimize environmental impact while maintaining the quality and scalability of the production process.
Future Directions in Graphene Research
Exploring graphene’s diverse properties, researchers are continually seeking innovative methodologies to enhance its applicability. Developing hybrid materials that integrate graphene with other nanostructures, such as quantum dots or metal nanoparticles, may lead to synergistic effects,contributing to enhanced functionalities in energy storage, sensing, and catalysis.
Conclusion
Graphene’s composition intrinsically links it to carbon atoms, forming a unique lattice structure that is pivotal to its remarkable properties. The various synthetic techniques harness the intrinsic nature of carbon to produce high-quality graphene for versatile applications. As the understanding of its properties deepens and production processes evolve, graphene holds the potential to revolutionize myriad technologies and industries. Future research will undoubtedly focus on optimizing the synthesis process while exploring new avenues for graphene integration across numerous fields. Ultimately, the relationship between graphene and carbon underscores the remarkable interplay of elemental composition and material properties, illuminating the path for next-generation advancements.