Carbon, the fourth most abundant element in the universe, is akin to the master sculptor of materials, shaping myriad forms and functionalities. Within this elemental gallery, carbon nanomaterials stand out as exquisite sculptures—intricate, nuanced, and, above all, profoundly versatile. These materials, ranging from graphene to carbon nanotubes, capture the imagination of researchers and industries alike with their remarkable properties and applications. Understanding the different types of carbon nanomaterials is akin to peeling back the layers of an onion, revealing the complexity and utility that lie within each layer.
At the heart of this discussion lies graphene, an atom-thick sheet of carbon atoms arranged in a hexagonal lattice. Often heralded as a ‘wonder material,’ graphene exhibits extraordinary mechanical strength, thermal conductivity, and electrical conductivity. It demonstrates a tensile strength over 100 times greater than steel, making it an unparalleled candidate for applications ranging from flexible electronics to advanced composite materials. The metaphor of a tightrope walker navigating between the void of strength and flexibility encapsulates graphene’s unique appeal; it is both delicate and remarkably resilient.
Next in the ensemble of carbon nanomaterials are carbon nanotubes (CNTs), cylindrical structures that can be thought of as rolled-up sheets of graphene. With their remarkable aspect ratios and astounding mechanical properties, carbon nanotubes have catalyzed innovations in nanotechnology. There are primarily two types of carbon nanotubes: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs, akin to fine thread spun from the essence of carbon, possess exceptional electrical properties, making them prime candidates for applications in nanoelectronics. Conversely, MWCNTs, resembling a bundle of juxtaposed cylinders, exhibit superior mechanical strength and thermal stability, presenting opportunities in composite materials for aerospace and automotive industries.
Beyond graphene and carbon nanotubes lie fullerene structures, whose spherical symmetry evokes the image of a geodesic dome meticulously crafted from carbon atoms. Fullerenes, with their varied shapes including C60 (buckyballs) and carbon nanotubes themselves, exhibit unique electronic properties. The buckyball, for instance, functions as a potential electron acceptor, transforming concepts in photovoltaics and drug delivery systems. Here, the metaphor of the chameleon becomes apt; fullerenes can transition between roles with ease, adapting to myriad functions that span medicine to material science.
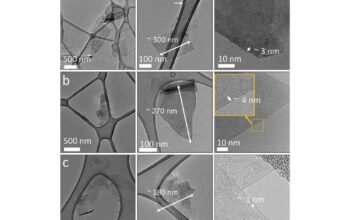
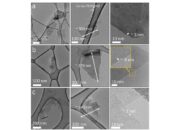
Further enriching this nano-gallery is carbon quantum dots (CQDs). These zero-dimensional nanoparticles exhibit quantum confinement effects that result in distinct optical properties, including photoluminescence. Their characteristic size falls below 10 nanometers, rendering them ideal candidates for applications in bioimaging, photonics, and light-emitting devices. CQDs can be likened to stars sprinkled across the firmament; despite their minuscule size, they illuminate potential avenues in nanotechnology, particularly in biomedical applications that necessitate high levels of sensitivity and specificity.
Graphene oxide (GO) and reduced graphene oxide (rGO) represent another fascinating bifurcation in carbon’s artistic expression. Graphene oxide, through oxidation, introduces oxygen-containing functional groups into the graphene lattice, thereby enhancing its hydrophilicity and making it amenable for various functionalization strategies. Its analog, reduced graphene oxide, emerges as a phoenix from the ashes of oxidation, reestablishing some of graphene’s pristine properties while maintaining a modulated degree of functionalization. The duality of GO and rGO elucidates a dialogue of transformation, emphasizing the fluidity of material properties, critical for applications in energy storage and sensing technologies.
Moreover, carbon-based nanocomposites amalgamate these nanomaterials with polymers or metals, much like a finely crafted mosaic where each piece contributes to a greater aesthetic and functional narrative. These composites exploit the superior properties of carbon nanomaterials to enhance mechanical, thermal, and electrical properties of the host matrix. This synergistic relationship rediscovers the ethos of collaboration, transforming conventional materials into something transcendent through the addition of carbon nanomaterials.
As one delves deeper into this fascinating world, the potential toxicological implications and environmental impacts of carbon nanomaterials warrant sober consideration. While the benefits are numerous, understanding the health implications remains paramount. The introduction of these particles into biological systems can elicit cellular responses that may differ remarkably from bulk materials. Engaging with this knowledge is akin to navigating a labyrinth; prudent investigation and responsible innovation are vital to harnessing the full potential of carbon nanomaterials while safeguarding human health.
In conclusion, the diverse array of carbon nanomaterials—from the resilient graphene and carbon nanotubes to the adaptable fullerenes and luminescent carbon quantum dots—paints a rich tapestry of material science. Each type unfolds layers of functionality and application, reinforcing the notion that carbon is not merely an element, but an architect of innovation. As research advances, the exploration of these captivating materials promises to unlock uncharted territories in nanotechnology, setting the stage for remarkable leaps in science and industry.