Graphene, a remarkable allotrope of carbon characterized by a two-dimensional hexagonal lattice, has captured the imagination of scientists and engineers due to its exceptional mechanical properties and potential applications in various fields, from electronics to nanotechnology. One inquiry that frequently arises in discussions surrounding this material is, “How much force is required to break graphene?” To unravel this question, one must delve into the fundamental aspects of graphene’s structure and the underlying physical principles that govern its strength.
To begin comprehending the force requisite to fracture graphene, it is crucial to understand its structural composition. Graphene is composed of a single layer of carbon atoms arranged in a honeycomb pattern, where each carbon atom is covalently bonded to three others, creating an extraordinarily strong and stable network. This arrangement not only contributes to its high tensile strength but also grants graphene an impressive Young’s modulus, which can reach upwards of 1 TPa (terapascal). This value places graphene among the strongest materials known, even more robust than steel by several orders of magnitude.
A pivotal aspect of understanding the force needed to break graphene lies in the concept of tensile strength, defined as the maximum amount of tensile (stretching) stress that a material can withstand before failure. For graphene, theoretical estimates suggest a tensile strength exceeding 130 GPa (gigapascals), contingent on the absence of defects or impurities, which can significantly weaken its intrinsic properties. Experimental investigations, however, have revealed slightly lower values, typically around 80–100 GPa, owing to real-world imperfections.
These figures compel a deeper examination of the force applied to a standard graphene sheet. Utilizing basic physics, one can derive the force required to break a sheet of graphene by employing the formula:
F = σ × A
Where F represents the force, σ symbolizes the tensile strength, and A denotes the cross-sectional area. A conventional graphene sheet measures approximately 1 square meter in area. By inputting the tensile strength the corresponding force can be calculated. For instance, if one considers a tensile strength of 100 GPa and an area of 1 m², the force exerted would be around 100 billion newtons, an unthinkably colossal amount.
This hypothetical calculation, while revealing, does not elucidate the full spectrum of factors influencing graphene’s fracture behavior. The presence and type of defects play a pivotal role in altering tensile strength. Defects such as vacancies, edges, and grain boundaries can significantly diminish the load capacity of graphene sheets. In real-world applications, graphene is rarely perfect. The inevitable introduction of such imperfections can lead to a heterogeneous distribution of stress during loading, culminating in premature failure.
Additionally, an array of external conditions can influence the mechanical properties of graphene. For instance, temperature fluctuations can affect bonding forces on the atomic level. Elevated temperatures may lead to reduced strength due to increased atomic mobility, whereas cryogenic temperatures could enhance material robustness. Environmental factors, such as humidity or exposure to different atmospheres, can also induce alterations in defect structures, subsequently affecting the force required for rupture.
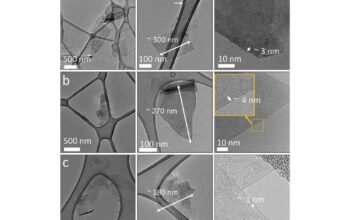
The method of graphene synthesis further infers on mechanical integrity, with techniques such as chemical vapor deposition (CVD), liquid-phase exfoliation, and mechanical exfoliation producing varied results in terms of defect density and material uniformity. CVD-grown graphene is often lauded for its high quality and minimal defects, leading to more accurate realizations of the theoretical tensile strength. On the contrary, liquid-phase exfoliated graphene frequently exhibits a significant number of defects, resulting in weaker material compared to its pristine counterpart.
Another consideration is the role of size and scale. Experiments have indicated a size-dependent effect in two-dimensional materials, where smaller samples showcase different mechanical properties than larger ones due to surface effects taking precedence. In this context, as the lateral dimensions of graphene sheets decrease, the likelihood of encountering defects may increase, thereby necessitating a reconsideration of the force required to break a small sheet versus a macroscopic sample.
The exploration of force and its effects on graphene extends beyond mere academic scrutiny. The fascination with such a material stems not only from its structural capabilities but also from the potential applications that could orbit around its use. Researchers are fervently probing into utilizing graphene in flexible electronics, advanced composites, and even biomedical devices. These applications necessitate a thorough understanding of graphene’s mechanical limits, as knowledge of the amount of force required to break it informs design parameters in real-world applications.
In summation, the inquiry into how much force it takes to break graphene unveils a tapestry of concepts that intertwine the macroscale with the nanoscale. As one navigates through the realms of tensile strength, molecular defects, external influences, synthesis methods, and size effects, it becomes apparent that the force required for fracture is not merely a static value but a dynamic characteristic influenced by multifaceted parameters. The ongoing quest to harness the full potential of graphene will require a continuous reevaluation of these factors, ultimately leading to innovations that capitalize on the extraordinary attributes of this remarkable material.