Graphene, a one-atom-thick layer of carbon atoms arranged in a two-dimensional honeycomb lattice, has garnered significant interest in the scientific community. Its remarkable electrical, thermal, and mechanical properties position it as a potential game-changer in numerous fields, from electronics to materials science. However, beneath this veneer of promise lies a fundamental issue: why is graphene often deemed unstable? This inquiry invites further exploration into the subtleties of graphene’s intrinsic characteristics, external influences, and the implications of its behavior in various environments.
To frame the discussion, it is essential to comprehend the conceptual stability of graphene. On a theoretical level, graphene exhibits exceptional stability due to its robust sp2 hybridization. Each carbon atom’s electron configuration allows it to form strong covalent bonds with neighboring atoms, which theoretically renders it insusceptible to decomposition. Yet, this perception of stability is mitigated in practical applications.
One significant challenge arises from the propensity of graphene to react with environmental factors. Encounters with oxygen, moisture, and other reactive species can catalyze processes leading to degradation. For instance, the oxidative degradation can compromise the integrity of the graphene lattice, resulting in the formation of oxygen-containing functional groups that disrupt its electronic characteristics. Such modifications not only alter electrical conductivity but also hinder the material’s mechanical properties.
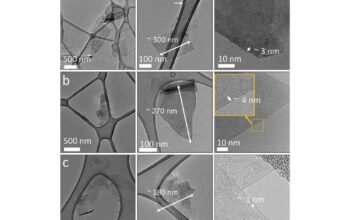
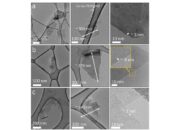
Moreover, defects within the graphene lattice contribute significantly to its instability. Defects can arise during fabrication processes or from exposure to harsh conditions. Point defects, such as vacancies or substitutional atoms, induce localized strain, thereby creating regions of weakness within the otherwise uniformly structured lattice. Such anomalies can facilitate further reactivity, leading to the proliferation of additional defects and a potential cascade of structural failure.
To pose a playful yet thought-provoking question: can the very attributes that grant graphene its impressive capabilities also render it more vulnerable to instability? Indeed, delving into the extremes of its atomic structure, we find that the very thinness of the graphene layer, while advantageous for applications requiring minimal mass or weight, also makes it prone to physical disturbances, such as mechanical stress or nanoscale tears.
Another qualitative aspect seminal to graphene’s instability is its interaction with substrates. When applied as a coating or layer upon other materials, these interactions can invoke substantial modifications to its properties. The nature of the substrate can introduce additional forces that either reinforce or compromise graphene’s structure. When these two-dimensional sheets adhere to specific surfaces, interfacial forces may exasperate the likelihood of fracturing or delaminating under stress or thermal variation.
Furthermore, temperature effects play a pivotal role in determining the stability of graphene. As temperature escalates, the vibrational modes of the carbon atoms in the lattice intensify, which can, in turn, exacerbate thermal expansion and contraction. This thermal fluctuation may lead to stress accumulation in the material, culminating in structural failure. An exploration into the thermal stability of graphene elucidates a paradox; while it can withstand elevated temperatures in controlled environments, exposure to sudden temperature shifts or erratic thermal events can precipitate instability.
Additionally, the electronic properties of graphene are sensitive to its chemical milieu. When doped with various substances, the electronic band structure is modified, which can lead to catastrophic failure in devices relying upon specific electronic characteristics. This phenomenon is intrinsically linked to the material’s destiny as an ideal conductor and signifies a compelling intersection of chemistry and physics that shapes graphene’s applications.
While addressing the challenges associated with graphene stability, it is prudent to consider advancements in remediation and synthesis techniques. Emerging methodologies, such as chemical vapor deposition (CVD), aim to produce higher-quality graphene with fewer defects and enhanced uniformity. Innovations like these present opportunities to mitigate the deleterious effects of imperfections and environmental interactions, proffering a glimmer of hope for the future scalability of graphene-based technologies.
Despite the aforementioned challenges, it is crucial to recognize that instability does not equate to obsolescence. Instead, it motivates vigorous research into stabilization strategies. The development of hybrid materials where graphene is integrated with other compounds may offer synergistic benefits. For instance, embedding graphene within a polymer matrix can enhance its overall mechanical stability while retaining the advantageous properties of graphene.
In conclusion, while graphene’s innate characteristics afford it exceptional properties, various factors contribute to its instability, ranging from environmental interactions and defect introduction to substrate effects and thermal fluctuations. This understanding of graphene’s instabilities not only elucidates fundamental scientific principles but also paves the way for innovative solutions to harness its unique benefits. As research continues to evolve, the interplay between stability and reactivity will offer a rich landscape for exploration, encompassing both challenges and opportunities in the quest for advanced materials.