When considering the electrical conductivity of materials, one might ponder: Is inorganic graphite a good conductor of electricity? This seemingly straightforward inquiry delves into the realms of material science and solid-state physics, unearthing a plethora of intriguing phenomena associated with graphite’s unique structural composition. The physical characteristics of graphite—which is composed of sheets of carbon atoms bonded in a hexagonal lattice—offer a fascinating glimpse into its electrical properties, making this topic ripe for exploration.
Graphite is classified as a form of carbon, a quintessential element that plays a pivotal role in various domains, including chemistry, materials science, and electronics. It is essential to distinguish between organic and inorganic forms of graphite. Organic graphite typically pertains to naturally occurring graphite, while inorganic graphite encompasses synthetically derived forms. The focus here will be on inorganic graphite, as it presents distinctive characteristics. But does it fulfill the criteria for being labeled a good electrical conductor?
To comprehensively investigate this query, one must first grasp the fundamental principles of electrical conductivity. The ability of a material to conduct electricity hinges on the presence of free charge carriers—typically electrons or holes—that can move through its structure. In metals, a dense sea of delocalized electrons facilitates rapid conduction. Conversely, insulators essentially lack these free charge carriers, resulting in a poor ability to conduct electricity.
Graphite presents a hybrid scenario. Its crystalline structure is characterized by layers of graphene—two-dimensional arrays of carbon atoms—with weak van der Waals forces holding these layers together. The electrons within each graphene layer can move freely, akin to their metallic counterparts. This movement of electrons is primarily responsible for graphite’s ability to conduct electricity. Intriguingly, the layered structure allows for excellent in-plane conductivity while exhibiting comparatively low electrical conductivity perpendicular to the layers. Hence, graphite can be classified as an anisotropic conductor, its conductive prowess varying with orientation.
The peculiar arrangement of carbon atoms in graphite imparts additional complexities. The presence of π-bonds formed by the overlap of p-orbitals gives rise to a unique electronic band structure. The conduction band is partially filled, which facilitates electron mobility. Consequently, when an external electric field is applied, electrons can migrate throughout the lattice, allowing graphite to readily conduct electricity. This phenomenon highlights why graphite is oftentimes employed in applications such as electrodes in batteries and capacitors, where efficient electrical conduction is paramount.
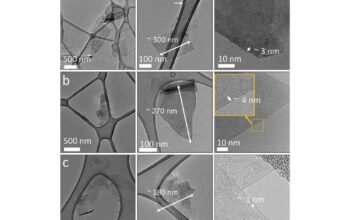
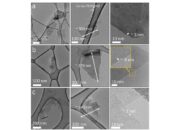
However, the story of graphite’s conductivity does not conclude with its structural advantages. Various extrinsic factors can significantly influence its conductive capacity. For instance, the purity of graphite plays a crucial role. Impurities and defects within the crystalline lattice can scatter charge carriers, thereby diminishing conductivity. Hence, high-purity synthetic graphite is widely sought after in electronics to ensure optimal performance.
Another noteworthy aspect is temperature dependency. Graphite’s electrical conductivity tends to increase with rising temperatures, a trend that’s atypical compared to metals where conductivity usually decreases with temperature. At elevated temperatures, the thermal energy enhances the lattice vibrations, which can assist in overcoming barriers that impede electron flow, thereby facilitating conductivity. This behavior may also indicate the potential for new applications of graphite in thermoelectric materials, where temperature-driven electricity generation is essential.
Interestingly, while graphite exhibits commendable conductivity, it pales in comparison to some metals. Silver, copper, and gold are stalwarts in the conduction arena, boasting superior conductivity due to their densely packed electron structure. Despite this, graphite’s unique combination of attributes—lightweight, chemically stable, and amenable to various modifications—renders it an appealing candidate for specific applications where metal conductivity may not be advantageous.
Moreover, the burgeoning field of nanotechnology has elicited renewed interest in the electrical properties of modified graphite materials, such as graphene and carbon nanotubes. These materials showcase remarkable electrical characteristics, offering pathways for the design of next-generation electronic devices. Graphene, in particular, has garnered attention for its exceptional conductivity and mechanical strength, presenting exciting prospects for innovation and application beyond conventional graphite.
In summary, the question “Is inorganic graphite a good conductor of electricity?” can be answered affirmatively, albeit with a nuanced understanding. The layered hexagonal framework, coupled with the unique electronic properties imparted by the bonding structure, elucidates why inorganic graphite possesses substantial conductivity. Yet, it is vital to contextualize its conductive abilities relative to other materials, taking into account factors such as purity and temperature effects. Additionally, the evolution of conductive graphite applications in emerging technologies signifies its invaluable role in the future of material science.
Ultimately, as research continues to evolve, further explorations of inorganic graphite may yield innovative applications that challenge traditional notions of conductivity, reinventing our understanding of this ever-relevant material.